High-Intensity Interval Training Attenuates Ketogenic Diet-Induced Liver Fibrosis in Type 2 Diabetic Mice by Ameliorating TGF-β1/Smad Signaling
- PMID: 33192083
- PMCID: PMC7656782
- DOI: 10.2147/DMSO.S275660
High-Intensity Interval Training Attenuates Ketogenic Diet-Induced Liver Fibrosis in Type 2 Diabetic Mice by Ameliorating TGF-β1/Smad Signaling
Abstract
Objective: Ketogenic diet (KD) and high-intensity interval training (HIIT) have preclinical benefits for type 2 diabetes (Db). However, the health risks of long-term KD use in diabetes should be ascertained and prevented. We hypothesized that KD-induced liver fibrosis in type 2 diabetic mice could be ameliorated by HIIT.
Methods: Streptozotocin-induced type 2 diabetic mice were divided into high-fat diet (HFD) control (Db+HFD+Sed), KD control (Db+KD+Sed), HFD coupled with HIIT (Db+HFD+HIIT), and KD coupled with HIIT (Db+KD+HIIT) groups (n=6, per group). Control mice were kept in sedentary (Sed), while HIIT group mice underwent 40-minute high-intensity interval training three alternate days per week. After 8-week intervention, the indicators of body weight and insulin resistance, oxidative stress markers, hepatic fibrosis, genetic and protein expression of related pathways were tested.
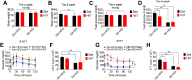
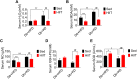
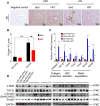
Results: We found that fasting blood glucose level was reduced in the Db+HFD+HIIT, Db+KD+Sed, and Db+KD+HIIT groups. Insulin sensitivity was increased in diabetic mice of these groups, whereas ROS levels were decreased in mice that underwent HIIT. The immunohistochemical staining of liver, serum index, and hepatic parameters of diabetic mice in the KD group revealed liver fibrosis, which was significantly attenuated by HIIT. Besides, these effects of HIIT were the outcome of hepatic stellate cell's inactivation, reduced protein expression of matrix metalloproteinases and tissue inhibitor of metalloproteinases, and the inhibition of TGF-β1/Smad signaling.
Conclusion: KD had a profound fibrotic effect on the liver of type 2 diabetic mice, whereas HIIT ameliorated this effect. KD did not show any apparent benefit as far as glucose tolerance and homeostasis were concerned. Concisely, our results demonstrated that KD should be coupled with HIIT for the prevention and preclinical mitigation of type 2 diabetes.
Keywords: ROS; TGF-β1/Smad signal; diabetes; hepatic fibrosis; high-intensity interval training; ketogenic diet.
© 2020 Zhang et al.
Conflict of interest statement
Dr. Yi Sun and Dr. ShuZhe Ding report grants from the National Natural Science Foundation of China, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

