LncRNA MALAT1 Promotes Survival of Epithelial Ovarian Cancer Cells by Downregulating miR-145-5p
- PMID: 33192095
- PMCID: PMC7654532
- DOI: 10.2147/CMAR.S267355
LncRNA MALAT1 Promotes Survival of Epithelial Ovarian Cancer Cells by Downregulating miR-145-5p
Retraction in
-
LncRNA MALAT1 Promotes Survival of Epithelial Ovarian Cancer Cells by Downregulating miR-145-5p [Retraction].Cancer Manag Res. 2021 Sep 19;13:7251-7252. doi: 10.2147/CMAR.S339843. eCollection 2021. Cancer Manag Res. 2021. PMID: 34584451 Free PMC article.
Abstract
Purpose: This paper was aimed at investigating the regulatory mechanism of long non-coding RNA metastasis-associated lung adenocarcinoma transcript-1 (MALAT1) in epithelial ovarian cancer (EOC).
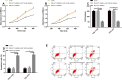
Materials and methods: MALAT1 and miR-145-5p expression in the tissues, serum, and EOC cell lines (TOV-112D, TOV-21G) of patients with EOC were detected. The two genes were transfected into the cells via upregulating or downregulating their expression. Levels of apoptosis-related proteins (Caspase-3, Bax, Bcl-2) were analyzed. Mechanisms of cell proliferation, invasion, and apoptosis were studied.
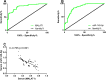
Results: MALAT1 was high expressed in EOC tissues, while miR-145-5p was poorly expressed in them. The areas under the curves (AUCs) of the two genes for diagnosing EOC were greater than 0.850, and the two had a significantly negative correlation. According to multivariate Cox regression analysis, high MALAT1 expression, tumor size, degree of differentiation, case staging, and lymph node metastasis were the independent risk factors affecting prognosis. The 5-year overall survival rate (OSR) of patients with low MALAT1 expression was remarkably higher than that of those with high expression. Overexpressing miR-145-5p and silencing MALAT1 could inhibit EOC cells from proliferating and invading, increase their apoptotic rate, and improve levels of the apoptosis-related proteins. After co-transfection with MALAT1-inhibitor + miR-145-5p-inhibitor, the proliferation and invasion of TOV-112D and TOV-21G cells were inhibited and the apoptotic rate rose more obviously. Inhibiting MALAT1 could increase miR-145-5p expression, thus inhibiting EOC cells from proliferating and invading and thereby increasing their apoptotic rate.
Conclusion: MALAT1 promotes EOC cells' survival by downregulating miR-145-5p so it may become a new direction for EOC diagnosis and gene therapy.
Keywords: EOC; MALAT1; cell survival; miR-145-5p.
© 2020 Wang et al.
Conflict of interest statement
The authors report no conflicts of interest for this work. Ye Zhao and Yi-Min Wang are co-corresponding author.
Figures





References
-
- Zhao L, Kong H, Sun H, Chen Z, Chen B, Zhou M. LncRNA-PVT1 promotes pancreatic cancer cells proliferation and migration through acting as a molecular sponge to regulate miR-448. J Cell Physiol. 2018;233:4044–4055. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

