Dopamine, Alpha-Synuclein, and Mitochondrial Dysfunctions in Parkinsonian Eyes
- PMID: 33192254
- PMCID: PMC7604532
- DOI: 10.3389/fnins.2020.567129
Dopamine, Alpha-Synuclein, and Mitochondrial Dysfunctions in Parkinsonian Eyes
Abstract
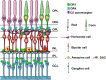
Parkinson's disease (PD) is characterized by motor dysfunctions including bradykinesia, tremor at rest and motor instability. These symptoms are associated with the progressive degeneration of dopaminergic neurons originating in the substantia nigra pars compacta and projecting to the corpus striatum, and by accumulation of cytoplasmic inclusions mainly consisting of aggregated alpha-synuclein, called Lewy bodies. PD is a complex, multifactorial disorder and its pathogenesis involves multiple pathways and mechanisms such as α-synuclein proteostasis, mitochondrial function, oxidative stress, calcium homeostasis, axonal transport, and neuroinflammation. Motor symptoms manifest when there is already an extensive dopamine denervation. There is therefore an urgent need for early biomarkers to apply disease-modifying therapeutic strategies. Visual defects and retinal abnormalities, including decreased visual acuity, abnormal spatial contrast sensitivity, color vision defects, or deficits in more complex visual tasks are present in the majority of PD patients. They are being considered for early diagnosis together with retinal imaging techniques are being considered as non-invasive biomarkers for PD. Dopaminergic cells can be found in the retina in a subpopulation of amacrine cells; however, the molecular mechanisms leading to visual deficits observed in PD patients are still largely unknown. This review provides a comprehensive analysis of the retinal abnormalities observed in PD patients and animal models and of the molecular mechanisms underlying neurodegeneration in parkinsonian eyes. We will review the role of α-synuclein aggregates in the retina pathology and/or in the onset of visual symptoms in PD suggesting that α-synuclein aggregates are harmful for the retina as well as for the brain. Moreover, we will summarize experimental evidence suggesting that the optic nerve pathology observed in PD resembles that seen in mitochondrial optic neuropathies highlighting the possible involvement of mitochondrial abnormalities in the development of PD visual defects. We finally propose that the eye may be considered as a complementary experimental model to identify possible novel disease' pathways or to test novel therapeutic approaches for PD.
Keywords: Parkinson’ disease; alpha-synuclein; dopamine; mitochondria; optic neuropathies; parkinsonism; retina; visual dysfunctions.
Copyright © 2020 Indrieri, Pizzarelli, Franco and De Leonibus.
Figures


References
-
- Ang M., Tan A. C. S., Cheung C. M. G., Keane P. A., Dolz-Marco R., Sng C. C. A., et al. (2018). Optical coherence tomography angiography: a review of current and future clinical applications. Graefes Arch. Clin. Exp. Ophthalmol. 256 237–245. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

