OGT-Mediated KEAP1 Glycosylation Accelerates NRF2 Degradation Leading to High Phosphate-Induced Vascular Calcification in Chronic Kidney Disease
- PMID: 33192538
- PMCID: PMC7649800
- DOI: 10.3389/fphys.2020.01092
OGT-Mediated KEAP1 Glycosylation Accelerates NRF2 Degradation Leading to High Phosphate-Induced Vascular Calcification in Chronic Kidney Disease
Retraction in
-
Retraction: OGT-mediated KEAP1 glycosylation accelerates NRF2 degradation leading to high phosphate-induced vascular calcification in chronic kidney disease.Front Physiol. 2024 Oct 9;15:1505634. doi: 10.3389/fphys.2024.1505634. eCollection 2024. Front Physiol. 2024. PMID: 39444754 Free PMC article.
Abstract
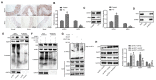
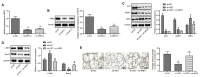
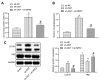
Unraveling the complex regulatory pathways that mediate the effects of phosphate on vascular smooth muscle cells (VSMCs) may provide novel targets and therapies to limit the destructive effects of vascular calcification (VC) in patients with chronic kidney disease (CKD). Our previous studies have highlighted several signaling networks associated with VSMC autophagy, but the underlying mechanisms remain poorly understood. Thereafter, the current study was performed to characterize the functional relevance of O-linked N-acetylglucosamine (GlcNAc) transferase (OGT) in high phosphate-induced VC in CKD settings. We generated VC models in 5/6 nephrectomized rats in vivo and VSMC calcification models in vitro. Artificial modulation of OGT (knockdown and overexpression) was performed to explore the role of OGT in VSMC autophagy and VC in thoracic aorta, and in vivo experiments were used to substantiate in vitro findings. Mechanistically, co-immunoprecipitation (Co-IP) assay was performed to examine interaction between OGT and kelch like ECH associated protein 1 (KEAP1), and in vivo ubiquitination assay was performed to examine ubiquitination extent of nuclear factor erythroid 2-related factor 2 (NRF2). OGT was highly expressed in high phosphate-induced 5/6 nephrectomized rats and VSMCs. OGT silencing was shown to suppress high phosphate-induced calcification of VSMCs. OGT enhances KEAP1 glycosylation and thereby results in degradation and ubiquitination of NRF2, concurrently inhibiting VSMC autophagy to promote VSMC calcification in 5/6 nephrectomized rats. OGT inhibits VSMC autophagy through the KEAP1/NRF2 axis and thus accelerates high phosphate-induced VC in CKD.
Keywords: O-linked N-acetylglucosamine transferase; autophagy; chronic kidney disease; high phosphorus; kelch like ECH associated protein 1; nuclear factor erythroid 2-related factor 2; vascular calcification; vascular smooth muscle cell.
Copyright © 2020 Xu, Du, Sheng, Li, Qiu, Tian and Yao.
Figures




References
-
- Andres-Bergos J., Tardio L., Larranaga-Vera A., Gomez R., Herrero-Beaumont G., Largo R. (2012). The increase in O-linked N-acetylglucosamine protein modification stimulates chondrogenic differentiation both in vitro and in vivo. J. Biol. Chem. 287, 33615–33628. 10.1074/jbc.M112.354241, PMID: - DOI - PMC - PubMed
-
- Cappato S., Tonachini L., Giacopelli F., Tirone M., Galietta L. J., Sormani M., et al. (2016). High-throughput screening for modulators of ACVR1 transcription: discovery of potential therapeutics for fibrodysplasia ossificans progressiva. Dis. Model. Mech. 9, 685–696. 10.1242/dmm.023929, PMID: - DOI - PMC - PubMed
-
- Chen P. H., Smith T. J., Wu J., Boyce M., Chi J. T. A., Chen P. H., et al. (2017a). Abstract 5457: OGT restrains the NRF2 antioxidant pathway via O-GlcNAcylation of KEAP1. Mol. Cell Biol. Genet. 77:5457. 10.1158/1538-7445.AM2017-5457 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

