The Mediator Subunit, Med23 Is Required for Embryonic Survival and Regulation of Canonical WNT Signaling During Cranial Ganglia Development
- PMID: 33192541
- PMCID: PMC7642510
- DOI: 10.3389/fphys.2020.531933
The Mediator Subunit, Med23 Is Required for Embryonic Survival and Regulation of Canonical WNT Signaling During Cranial Ganglia Development
Abstract
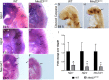
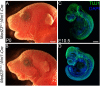
Development of the vertebrate head is a complex and dynamic process, which requires integration of all three germ layers and their derivatives. Of special importance are ectoderm-derived cells that form the cranial placodes, which then differentiate into the cranial ganglia and sensory organs. Critical to a fully functioning head, defects in cranial placode and sensory organ development can result in congenital craniofacial anomalies. In a forward genetic screen aimed at identifying novel regulators of craniofacial development, we discovered an embryonically lethal mouse mutant, snouty, which exhibits malformation of the facial prominences, cranial nerves and vasculature. The snouty mutation was mapped to a single nucleotide change in a ubiquitously expressed gene, Med23, which encodes a subunit of the global transcription co-factor complex, Mediator. Phenotypic analyses revealed that the craniofacial anomalies, particularly of the cranial ganglia, were caused by a failure in the proper specification of cranial placode neuronal precursors. Molecular analyses determined that defects in cranial placode neuronal differentiation in Med23 sn/sn mutants were associated with elevated WNT/β-catenin signaling, which can be partially rescued through combined Lrp6 and Wise loss-of-function. Our work therefore reveals a surprisingly tissue specific role for the ubiquitously expressed mediator complex protein Med23 in placode differentiation during cranial ganglia development. This highlights the importance of coupling general transcription to the regulation of WNT signaling during embryogenesis.
Keywords: MED23; Wnt signaling; cranial ganglia; cranial placodes; neural crest cells.
Copyright © 2020 Dash, Bhatt, Sandell, Seidel, Ahn, Krumlauf and Trainor.
Figures








References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

