The Role of Kv7.2 in Neurodevelopment: Insights and Gaps in Our Understanding
- PMID: 33192566
- PMCID: PMC7657400
- DOI: 10.3389/fphys.2020.570588
The Role of Kv7.2 in Neurodevelopment: Insights and Gaps in Our Understanding
Abstract
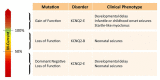
Kv7.2 subunits encoded by the KCNQ2 gene constitute a critical molecular component of the M-current, a subthreshold voltage-gated potassium current controlling neuronal excitability by dampening repetitive action potential firing. Pathogenic loss-of-function variants in KCNQ2 have been linked to epilepsy since 1998, and there is ample functional evidence showing that dysfunction of the channel indeed results in neuronal hyperexcitability. The recent description of individuals with severe developmental delay with or without seizures due to pathogenic variants in KCNQ2 (KCNQ2-encephalopathy) reveals that Kv7.2 channels also have an important role in neurodevelopment. Kv7.2 channels are expressed already very early in the developing brain when key developmental processes such as proliferation, differentiation, and synaptogenesis play a crucial role in brain morphogenesis and maturation. In this review, we will discuss the available evidence for a role of Kv7.2 channels in these neurodevelopmental processes, focusing in particular on insights derived from KCNQ2-related human phenotypes, from the spatio-temporal expression of Kv7.2 and other Kv7 family member, and from cellular and rodent models, highlighting critical gaps and research strategies to be implemented in the future. Lastly, we propose a model which divides the M-current activity in three different developmental stages, correlating with the cell characteristics during these particular periods in neuronal development, and how this can be linked with KCNQ2-related disorders. Understanding these mechanisms can create opportunities for new targeted therapies for KCNQ2-encephalopathy.
Keywords: KCNQ2; KCNQ2-encephalopathy; Kv7.2; M-current; neurodevelopment.
Copyright © 2020 Dirkx, Miceli, Taglialatela and Weckhuysen.
Figures


References
-
- Abidi A., Devaux J. J., Molinari F., Alcaraz G., Michon F. X., Sutera-Sardo J., et al. . (2015). A recurrent KCNQ2 pore mutation causing early onset epileptic encephalopathy has a moderate effect on M current but alters subcellular localization of Kv7 channels. Neurobiol. Dis. 80, 80–92. 10.1016/j.nbd.2015.04.017, PMID: - DOI - PubMed
-
- Ambrosino P., Freri E., Castellotti B., Soldovieri M. V., Mosca I., Manocchio L., et al. . (2018). Kv7.3 compound heterozygous variants in early onset encephalopathy reveal additive contribution of C-terminal residues to PIP2-dependent K(+) channel gating. Mol. Neurobiol. 55, 7009–7024. 10.1007/s12035-018-0883-5, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

