Natural Killer Lytic-Associated Molecule (NKLAM): An E3 Ubiquitin Ligase With an Integral Role in Innate Immunity
- PMID: 33192571
- PMCID: PMC7658342
- DOI: 10.3389/fphys.2020.573372
Natural Killer Lytic-Associated Molecule (NKLAM): An E3 Ubiquitin Ligase With an Integral Role in Innate Immunity
Abstract
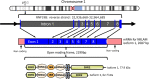
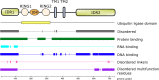
Natural Killer Lytic-Associated Molecule (NKLAM), also designated RNF19B, is a unique member of a small family of E3 ubiquitin ligases. This 14-member group of ligases has a characteristic cysteine-rich RING-IBR-RING (RBR) domain that mediates the ubiquitination of multiple substrates. The consequence of substrate ubiquitination varies, depending on the type of ubiquitin linkages formed. The most widely studied effect of ubiquitination of proteins is proteasome-mediated substrate degradation; however, ubiquitination can also alter protein localization and function. Since its discovery in 1999, much has been deciphered about the role of NKLAM in innate immune responses. We have discerned that NKLAM has an integral function in both natural killer (NK) cells and macrophages in vitro and in vivo. NKLAM expression is required for each of these cell types to mediate maximal killing activity and cytokine production. However, much remains to be determined. In this review, we summarize what has been learned about NKLAM expression, structure and function, and discuss new directions for investigation. We hope that this will stimulate interest in further exploration of NKLAM.
Keywords: NKLAM; RNF19B; cytotoxicity; innate immunity; macrophage; natural killer; phagocytosis; ubiquitin ligase.
Copyright © 2020 Lawrence, Willard, Cochran, Matchett and Kornbluth.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

