Postzygotic Somatic Mutations in the Human Brain Expand the Threshold-Liability Model of Schizophrenia
- PMID: 33192734
- PMCID: PMC7642466
- DOI: 10.3389/fpsyt.2020.587162
Postzygotic Somatic Mutations in the Human Brain Expand the Threshold-Liability Model of Schizophrenia
Abstract
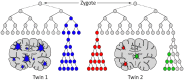
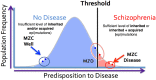
The search for what causes schizophrenia has been onerous. This research has included extensive assessment of a variety of genetic and environmental factors using ever emerging high-resolution technologies and traditional understanding of the biology of the brain. These efforts have identified a large number of schizophrenia-associated genes, some of which are altered by mutational and epi-mutational mechanisms in a threshold liability model of schizophrenia development. The results, however, have limited predictability and the actual cause of the disease remains unknown. This current state asks for conceptualizing the problem differently in light of novel insights into the nature of mutations, the biology of the brain and the fine precision and resolution of emerging technologies. There is mounting evidence that mutations acquired during postzygotic development are more common than germline mutations. Also, the postzygotic somatic mutations including epimutations (PZMs), which often lead to somatic mosaicism, are relatively common in the mammalian brain in comparison to most other tissues and PZMs are more common in patients with neurodevelopmental mental disorders, including schizophrenia. Further, previously inaccessible, detection of PZMs is becoming feasible with the advent of novel technologies that include single-cell genomics and epigenomics and the use of exquisite experimental designs including use of monozygotic twins discordant for the disease. These developments allow us to propose a working hypothesis and expand the threshold liability model of schizophrenia that already encompasses familial genetic, epigenetic and environmental factors to include somatic de novo PZMs. Further, we offer a test for this expanded model using currently available genome sequences and methylome data on monozygotic twins discordant for schizophrenia (MZD) and their parents. The results of this analysis argue that PZMs play a significant role in the development of schizophrenia and explain extensive heterogeneity seen across patients. It also offers the potential to convincingly link PZMs to both nervous system health and disease, an area that has remained challenging to study and relatively under explored.
Keywords: de novo mutations; epimutations; mosaicism; neurodevelopment; neurological disorders; neuronal diversity; postzygotic somatic mutations; threshold liability model.
Copyright © 2020 Singh, Castellani and Hill.
Figures


Comment in
-
Commentary on Singh et al. (2020) Postzygotic Somatic Mutations in the Human Brain Expand the Threshold-Liability Model of Schizophrenia.Front Psychiatry. 2021 Aug 6;12:653624. doi: 10.3389/fpsyt.2021.653624. eCollection 2021. Front Psychiatry. 2021. PMID: 34421665 Free PMC article. No abstract available.
References
Publication types
LinkOut - more resources
Full Text Sources

