Inhibitory Effect of Morin Against Candida albicans Pathogenicity and Virulence Factor Production: An in vitro and in vivo Approaches
- PMID: 33193145
- PMCID: PMC7644646
- DOI: 10.3389/fmicb.2020.561298
Inhibitory Effect of Morin Against Candida albicans Pathogenicity and Virulence Factor Production: An in vitro and in vivo Approaches
Abstract
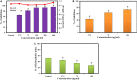
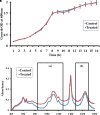
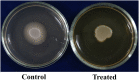
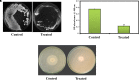
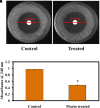
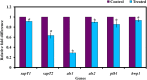
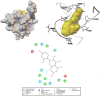
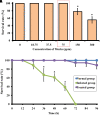
Candida albicans is considered an exclusive etiologic agent of candidiasis, a very common fungal infection in human. The expression of virulence factors contributes highly to the pathogenicity of C. albicans. These factors include biofilm formation, yeast-to-hyphal transition, adhesins, aspartyl proteases, and phospholipases secretion. Moreover, resistance development is a critical issue for the therapeutic failure of antifungal agents against systemic candidiasis. To circumvent resistance development, the present study investigated the virulence targeted therapeutic activity of the phyto-bioactive compound morin against C. albicans. Morin is a natural compound commonly found in medicinal plants and widely used in the pharmaceutical and cosmetic products/industries. The present study explicated the significant inhibitory potential of morin against biofilm formation and other virulence factors' production, such as yeast-hyphal formation, phospholipase, and exopolymeric substances, in C. albicans. Further, qPCR analysis confirmed the downregulation of biofilm and virlence-related genes in C. albicans upon morin treatment, which is in correspondence with the in vitro bioassays. Further, the docking analysis revealed that morin shows strong affinity with Hwp-1 protein, which regulates the expression of biofilm and hyphal formation in C. albicans and, thereby, abolishes fungal pathogenicity. Moreover, the anti-infective potential of morin against C. albicans-associated systemic candidiasis is confirmed through an in vivo approach using biomedical model organism zebrafish (Danio rerio). The outcomes of the in vivo study demonstrate that the morin treatment effectively rescues animals from C. albicans infections and extends their survival rate by inhibiting the internal colonization of C. albicans. Histopathology analysis revealed extensive candidiasis-related pathognomonic changes in the gills, intestine, and kidney of animals infected with C. albicans, while no extensive abnormalities were observed in morin-treated animals. The results evidenced that morin has the ability to protect against the pathognomonic effect and histopathological lesions caused by C. albicans infection in zebrafish. Thus, the present study suggests that the utilization of morin could act as a potent therapeutic medication for C. albicans instigated candidiasis.
Keywords: Candida albicans; anti-biofilm; anti-infective; anti-virulence; morin; systemic candidiasis; zebrafish.
Copyright © 2020 Abirami, Alexpandi, Durgadevi, Kannappan and Veera Ravi.
Figures


Similar articles
-
Anti-inflammatory potential of myristic acid and palmitic acid synergism against systemic candidiasis in Danio rerio (Zebrafish).Biomed Pharmacother. 2021 Jan;133:111043. doi: 10.1016/j.biopha.2020.111043. Epub 2020 Dec 1. Biomed Pharmacother. 2021. PMID: 33378951
-
Phloretin inhibited the pathogenicity and virulence factors against Candida albicans.Bioengineered. 2021 Dec;12(1):2420-2431. doi: 10.1080/21655979.2021.1933824. Bioengineered. 2021. PMID: 34167447 Free PMC article.
-
Pullulan nanoparticles inhibit the pathogenicity of Candida albicans by regulating hypha-related gene expression.Microbiol Spectr. 2024 Nov 14;12(12):e0104824. doi: 10.1128/spectrum.01048-24. Online ahead of print. Microbiol Spectr. 2024. PMID: 39540747 Free PMC article.
-
Suppression of hyphal formation and virulence of Candida albicans by natural and synthetic compounds.Biofouling. 2021 Jul;37(6):626-655. doi: 10.1080/08927014.2021.1948538. Epub 2021 Jul 20. Biofouling. 2021. PMID: 34284656 Review.
-
Virulence Factors in Candida species.Curr Protein Pept Sci. 2020;21(3):313-323. doi: 10.2174/1389203720666190722152415. Curr Protein Pept Sci. 2020. PMID: 31544690 Review.
Cited by
-
Novel Preclinical Study of Galloylquinic Acid Compounds from Copaifera lucens with Potent Antifungal Activity against Vaginal Candidiasis Induced in a Murine Model via Multitarget Modes of Action.Microbiol Spectr. 2022 Oct 26;10(5):e0272421. doi: 10.1128/spectrum.02724-21. Epub 2022 Aug 16. Microbiol Spectr. 2022. PMID: 35972130 Free PMC article.
-
Eugenol: A novel therapeutic agent for the inhibition of Candida species infection.Front Pharmacol. 2022 Aug 9;13:872127. doi: 10.3389/fphar.2022.872127. eCollection 2022. Front Pharmacol. 2022. PMID: 36016558 Free PMC article. Review.
-
The Bacillary Postbiotics, Including 2-Undecanone, Suppress the Virulence of Pathogenic Microorganisms.Pharmaceutics. 2022 Apr 29;14(5):962. doi: 10.3390/pharmaceutics14050962. Pharmaceutics. 2022. PMID: 35631548 Free PMC article.
-
Plant Preparations and Compounds with Activities against Biofilms Formed by Candida spp.J Fungi (Basel). 2021 May 5;7(5):360. doi: 10.3390/jof7050360. J Fungi (Basel). 2021. PMID: 34063007 Free PMC article. Review.
-
Systematic virtual screening in search of SARS CoV-2 inhibitors against spike glycoprotein: pharmacophore screening, molecular docking, ADMET analysis and MD simulations.Mol Divers. 2022 Oct;26(5):2775-2792. doi: 10.1007/s11030-022-10394-9. Epub 2022 Feb 8. Mol Divers. 2022. PMID: 35132518 Free PMC article.
References
-
- Alexpandi R., Prasanth M. I., Ravi A. V., Balamurugan K., Durgadevi R., Srinivasan R., et al. (2019). Protective effect of neglected plant Diplocyclos palmatus on quorum sensing mediated infection of Serratia marcescens and UV-A induced photoaging in model Caenorhabditis elegans. J. Photochem. Photobiol. B 201:111637. 10.1016/j.jphotobiol.2019.111637 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

