Diagnosis and Management of Hematological Adverse Events Induced by Immune Checkpoint Inhibitors: A Systematic Review
- PMID: 33193289
- PMCID: PMC7640759
- DOI: 10.3389/fimmu.2020.01354
Diagnosis and Management of Hematological Adverse Events Induced by Immune Checkpoint Inhibitors: A Systematic Review
Abstract
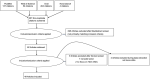
There has been less volume of literature focusing on the Immune-related Hematological Adverse Drug Events (Hem-irAEs) of Immune Checkpoint Inhibitors (ICPis) in cancer patients. Furthermore, there has been no consensus about the management of hematological toxicity from immunotherapy in the recently published practice guidelines by the European Society for Medical Oncology (ESMO). We conducted a systematic review of case reports/series to describe the diagnosis and management of potentially rare and unrecognized Hem-irAEs. We searched Medline, OVID, Web of Science for eligible articles. Data were extracted on patient characteristics, Hem-irAEs, and management strategies. We performed quality assessment using the Pierson-5 evaluation scheme and causality assessment using the Naranjo scale. Our search retrieved 49 articles that described 118 cases. The majority of patients had melanoma (57.6%) and lung cancer (26.3%). The most common Hem-irAEs reported with ICPis (such as nivolumab, ipilimumab, and pembrolizumab) were thrombocytopenia, hemolytic and aplastic anemias. Less reported adverse events included agranulocytosis and neutropenia. Steroids were commonly used to treat these adverse events with frequent success. Other used strategies included intravenous immunoglobulins (IVIG), rituximab, and transfusion of blood components. The findings of this review provide more insights into the diagnosis and management of the rarely reported Hem-irAEs of ICPis.
Keywords: atezolizumab; avelumab; durvalumab; immune checkpoint inhibitors; immune-related adverse events; ipilimumab; nivolumab; pembrolizumab.
Copyright © 2020 Omar, El-Fass, Abushouk, Elbaghdady, Barakat, Noreldin, Johar, Yassin, Hamad, Elazzazy and Dermime.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical