The Antigen Presenting Potential of CD21low B Cells
- PMID: 33193306
- PMCID: PMC7609862
- DOI: 10.3389/fimmu.2020.535784
The Antigen Presenting Potential of CD21low B Cells
Abstract
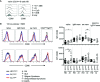
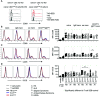
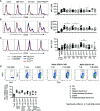
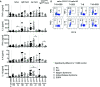
Human CD21low B cells are expanded in autoimmune (AI) diseases and display a unique phenotype with high expression of co-stimulatory molecules, compatible with a potential role as antigen-presenting cells (APCs). Thus, we addressed the co-stimulatory capacity of naïve-like, IgM-memory, switched memory and CD27negIgDneg memory CD21low B cells in allogenic co-cultures with CD4 T cells. CD21low B cells of patients with AI disorders expressed high levels of not only CD86, CD80, and HLA-DR (memory B cells) but also PD-L1 ex vivo and efficiently co-stimulated CD4 T cells of healthy donors (HD), as measured by upregulation of CD25, CD69, inducible co-stimulator (ICOS), and programmed cell death protein 1 (PD-1) and induction of cytokines. While the co-stimulatory capacity of the different CD21low B-cell populations was over all comparable to CD21pos counterparts of patients and HD, especially switched memory CD21low B cells lacked the increased capacity of CD21pos switched memory B-cells to induce high expression of ICOS, IL-2, IL-10, and IFN-γ. Acknowledging the limitation of the in vitro setting, CD21low B cells do not seem to preferentially support a specific Th effector response. In summary, our data implies that CD21low B cells of patients with AI diseases can become competent APCs and may, when enriched for autoreactive B-cell receptors (BCR), potentially contribute to AI reactions as cognate interaction partners of autoreactive T cells at sites of inflammation.
Keywords: CD21low B cells; T cell; antigen-presenting cells; autoimmunity; co-stimulation.
Copyright © 2020 Reincke, Payne, Harder, Strohmeier, Voll, Warnatz and Keller.
Figures

Similar articles
-
Low percentages of regulatory T cells in common variable immunodeficiency (CVID) patients with autoimmune diseases and its association with increased numbers of CD4+CD45RO+ T and CD21low B cells.Allergol Immunopathol (Madr). 2019 Sep-Oct;47(5):457-466. doi: 10.1016/j.aller.2019.01.003. Epub 2019 May 15. Allergol Immunopathol (Madr). 2019. PMID: 31103252
-
CD21(-/low) B cells in human blood are memory cells.Clin Exp Immunol. 2016 Aug;185(2):252-62. doi: 10.1111/cei.12795. Epub 2016 May 13. Clin Exp Immunol. 2016. PMID: 27010233 Free PMC article.
-
Age-Associated B Cells with Proinflammatory Characteristics Are Expanded in a Proportion of Multiple Sclerosis Patients.J Immunol. 2016 Dec 15;197(12):4576-4583. doi: 10.4049/jimmunol.1502448. Epub 2016 Nov 11. J Immunol. 2016. PMID: 27837111
-
Expression and role of CR1 and CR2 on B and T lymphocytes under physiological and autoimmune conditions.Mol Immunol. 2009 Sep;46(14):2767-73. doi: 10.1016/j.molimm.2009.05.181. Epub 2009 Jun 25. Mol Immunol. 2009. PMID: 19559484 Review.
-
A close-up on the expanding landscape of CD21-/low B cells in humans.Clin Exp Immunol. 2022 Dec 31;210(3):217-229. doi: 10.1093/cei/uxac103. Clin Exp Immunol. 2022. PMID: 36380692 Free PMC article. Review.
Cited by
-
Expansion of CD57+ CD8 T cells in common variable immunodeficiency with hepatopathy and CMV infection.Front Immunol. 2025 May 27;16:1577934. doi: 10.3389/fimmu.2025.1577934. eCollection 2025. Front Immunol. 2025. PMID: 40496855 Free PMC article.
-
Atypical phenotype and response of B cells in patients with seropositive rheumatoid arthritis.Clin Exp Immunol. 2021 May;204(2):221-238. doi: 10.1111/cei.13576. Epub 2021 Mar 2. Clin Exp Immunol. 2021. PMID: 33459349 Free PMC article.
-
Immune cell subsets in autoimmune polyendocrine syndrome type I.Sci Rep. 2025 Aug 4;15(1):28398. doi: 10.1038/s41598-025-12634-y. Sci Rep. 2025. PMID: 40759705 Free PMC article.
-
Imbalance of B-Cell Subpopulations in the Microenvironment of Sarcoidosis or Lung Cancer.Cells. 2024 Jul 29;13(15):1274. doi: 10.3390/cells13151274. Cells. 2024. PMID: 39120304 Free PMC article.
-
Targeting B cells and plasma cells in autoimmune diseases: From established treatments to novel therapeutic approaches.Eur J Immunol. 2023 Jan;53(1):e2149675. doi: 10.1002/eji.202149675. Epub 2022 Nov 16. Eur J Immunol. 2023. PMID: 36314264 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

