The Crotoxin:SBA-15 Complex Down-Regulates the Incidence and Intensity of Experimental Autoimmune Encephalomyelitis Through Peripheral and Central Actions
- PMID: 33193433
- PMCID: PMC7655790
- DOI: 10.3389/fimmu.2020.591563
The Crotoxin:SBA-15 Complex Down-Regulates the Incidence and Intensity of Experimental Autoimmune Encephalomyelitis Through Peripheral and Central Actions
Abstract
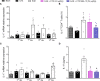
Crotoxin (CTX), the main neurotoxin from Crotalus durissus terrificus snake venom, has anti-inflammatory, immunomodulatory and antinociceptive activities. However, the CTX-induced toxicity may compromise its use. Under this scenario, the use of nanoparticle such as nanostructured mesoporous silica (SBA-15) as a carrier might become a feasible approach to improve CTX safety. Here, we determined the benefits of SBA-15 on CTX-related neuroinflammatory and immunomodulatory properties during experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis that replicates several histopathological and immunological features observed in humans. We showed that a single administration of CTX:SBA-15 (54 μg/kg) was more effective in reducing pain and ameliorated the clinical score (motor impairment) in EAE animals compared to the CTX-treated EAE group; therefore, improving the disease outcome. Of interest, CTX:SBA-15, but not unconjugated CTX, prevented EAE-induced atrophy and loss of muscle function. Further supporting an immune mechanism, CTX:SBA-15 treatment reduced both recruitment and proliferation of peripheral Th17 cells as well as diminished IL-17 expression and glial cells activation in the spinal cord in EAE animals when compared with CTX-treated EAE group. Finally, CTX:SBA-15, but not unconjugated CTX, prevented the EAE-induced cell infiltration in the CNS. These results provide evidence that SBA-15 maximizes the immunomodulatory and anti-inflammatory effects of CTX in an EAE model; therefore, suggesting that SBA-15 has the potential to improve CTX effectiveness in the treatment of MS.
Keywords: IL-17; SBA-15; crotoxin; experimental autoimmune encephalomyelitis (EAE); mesoporous silica; motor impairment; neuroinflammation.
Copyright © 2020 Sant'Anna, Giardini, Ribeiro, Lopes, Teixeira, Kimura, Bufalo, Ribeiro, Borrego, Cabrera, Ferreira, Zambelli, Sant'Anna and Picolo.
Figures


References
-
- MS Symptoms | Multiple Sclerosis Internation Federation Available at: https://www.msif.org/about-ms/symptoms-of-ms/ (Accessed March 25, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

