Roles of HLA-G in the Maternal-Fetal Immune Microenvironment
- PMID: 33193435
- PMCID: PMC7642459
- DOI: 10.3389/fimmu.2020.592010
Roles of HLA-G in the Maternal-Fetal Immune Microenvironment
Abstract
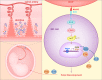
During pregnancy, the maternal uterus and fetus form a special microenvironment at the maternal-fetal interface to support fetal development. Extravillous trophoblasts (EVTs), differentiated from the fetus, invade into the decidua and interact with maternal cells. Human leukocyte antigen (HLA)-G is a non-classical MHC-I molecule that is expressed abundantly and specifically on EVTs in physiological conditions. Soluble HLA-G (sHLA-G) is also found in maternal blood, amniotic fluid, and cord blood. The abnormal expression and polymorphisms of HLA-G are related to adverse pregnancy outcomes such as preeclampsia (PE) and recurrent spontaneous abortion (RSA). Here we summarize current findings about three main roles of HLA-G during pregnancy, namely its promotion of spiral artery remodeling, immune tolerance, and fetal growth, all resulting from its interaction with immune cells. These findings are not only of great significance for the treatment of pregnancy-related diseases but also provide clues to tumor immunology research since HLA-G functions as a checkpoint in tumors.
Keywords: extravillous trophoblasts; fetal development; human leukocyte antigen G; immunology; natural killer cells; pregnancy; spiral artery remodeling.
Copyright © 2020 Xu, Zhou and Wei.
Figures


References
-
- Fujii T, Ishitani A, Geraghty DE. A Soluble Form of the Hla-G Antigen Is Encoded by a Messenger-Ribonucleic-Acid Containing Intron 4. J Immunol (1994) 153(12):5516–24. - PubMed
-
- Paul P, Cabestre FA, Ibrahim E, Lefebvre S, Khalil-Daher I, Vazeux G, et al. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol (2000) 61(11):1138–49. 10.1016/S0198-8859(00)00197-X - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

