Accumulation of Target Gene Mutations Confers Multiple Resistance to ALS, ACCase, and EPSPS Inhibitors in Lolium Species in Chile
- PMID: 33193482
- PMCID: PMC7655540
- DOI: 10.3389/fpls.2020.553948
Accumulation of Target Gene Mutations Confers Multiple Resistance to ALS, ACCase, and EPSPS Inhibitors in Lolium Species in Chile
Abstract
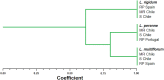
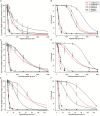
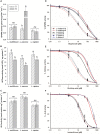
Different Lolium species, common weeds in cereal fields and fruit orchards in Chile, were reported showing isolated resistance to the acetyl CoA carboxylase (ACCase), acetolactate synthase (ALS) and 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) inhibiting herbicides in the late 1990s. The first case of multiple resistance to these herbicides was Lolium multiflorum found in spring barley in 2007. We hypothesized that other Lolium species may have evolved multiple resistance. In this study, we characterized the multiple resistance to glyphosate, diclofop-methyl and iodosulfuron-methyl-sodium in Lolium rigidum, Lolium perenne and Lolium multiflorum resistant (R) populations from Chile collected in cereal fields. Lolium spp. populations were confirmed by AFLP analysis to be L. rigidum, L. perenne and L. multiflorum. Dose-response assays confirmed multiple resistance to glyphosate, diclofop-methyl and iodosulfuron methyl-sodium in the three species. Enzyme activity assays (ACCase, ALS and EPSPS) suggested that the multiple resistance of the three Lolium spp. was caused by target site mechanisms, except the resistance to iodosulfuron in the R L. perenne population. The target site genes sequencing revealed that the R L. multiflorum population presented the Pro-106-Ser/Ala (EPSPS), Ile-2041-Asn++Asp-2078-Gly (ACCase), and Trp-574-Leu (ALS) mutations; and the R L. rigidum population had the Pro-106-Ser (EPSPS), Ile-1781-Leu+Asp-2078-Gly (ACCase) and Pro-197-Ser/Gln+Trp-574-Leu (ALS) mutations. Alternatively, the R L. perenne population showed only the Asp-2078-Gly (ACCase) mutation, while glyphosate resistance could be due to EPSPS gene amplification (no mutations but high basal enzyme activity), whereas iodosulfuron resistance presumably could involve non-target site resistance (NTSR) mechanisms. These results support that the accumulation of target site mutations confers multiple resistance to the ACCase, ALS and EPSPS inhibitors in L. multiflorum and L. rigidum from Chile, while in L. perenne, both target and NTSR could be present. Multiple resistance to three herbicide groups in three different species of the genus Lolium in South America represents a significant management challenge.
Keywords: diclofop-methyl; glyphosate; iodosulfuron methyl-sodium; italian ryegrass; perennial ryegrass; rigid ryegrass.
Copyright © 2020 Vázquez-García, Alcántara-de la Cruz, Palma-Bautista, Rojano-Delgado, Cruz-Hipólito, Torra, Barro and De Prado.
Figures
References
-
- Alcántara-de la Cruz R., Oliveira G. M., Carvalho L. B., Silva M. F. G. F. (2020). “Herbicide resistance in Brazil: status, impacts, and future challenges,” in Herbicides–Current Research and Case Studies in Use, 2nd Edn, ed. Ferreira K. M. (Rijeka: IntechOpen; ), 1–25. 10.5772/intechopen.91236 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

