Understanding Dietary Intervention-Mediated Epigenetic Modifications in Metabolic Diseases
- PMID: 33193730
- PMCID: PMC7593700
- DOI: 10.3389/fgene.2020.590369
Understanding Dietary Intervention-Mediated Epigenetic Modifications in Metabolic Diseases
Abstract
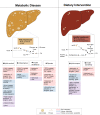
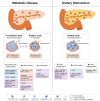
The global prevalence of metabolic disorders, such as obesity, diabetes and fatty liver disease, is dramatically increasing. Both genetic and environmental factors are well-known contributors to the development of these diseases and therefore, the study of epigenetics can provide additional mechanistic insight. Dietary interventions, including caloric restriction, intermittent fasting or time-restricted feeding, have shown promising improvements in patients' overall metabolic profiles (i.e., reduced body weight, improved glucose homeostasis), and an increasing number of studies have associated these beneficial effects with epigenetic alterations. In this article, we review epigenetic changes involved in both metabolic diseases and dietary interventions in primary metabolic tissues (i.e., adipose, liver, and pancreas) in hopes of elucidating potential biomarkers and therapeutic targets for disease prevention and treatment.
Keywords: DNA methylation; caloric restriction; dietary interventions; histone modification; intermittent fasting; non-alcoholic fatty liver disease; obesity; type 2 diabetes.
Copyright © 2020 Asif, Morrow, Mulvihill and Kim.
Figures




References
-
- Ahima R. S., Flier J. S. (2000). Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 11 327–332. - PubMed
-
- Ahrens M., Ammerpohl O., Von Schonfels W., Kolarova J., Bens S., Itzel T., et al. (2013). DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 18 296–302. 10.1016/j.cmet.2013.07.004 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

