Multifaceted Actions of GFI1 and GFI1B in Hematopoietic Stem Cell Self-Renewal and Lineage Commitment
- PMID: 33193732
- PMCID: PMC7649360
- DOI: 10.3389/fgene.2020.591099
Multifaceted Actions of GFI1 and GFI1B in Hematopoietic Stem Cell Self-Renewal and Lineage Commitment
Abstract
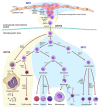
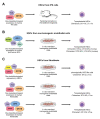
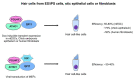
Growth factor independence 1 (GFI1) and the closely related protein GFI1B are small nuclear proteins that act as DNA binding transcriptional repressors. Both recognize the same consensus DNA binding motif via their C-terminal zinc finger domains and regulate the expression of their target genes by recruiting chromatin modifiers such as histone deacetylases (HDACs) and demethylases (LSD1) by using an N-terminal SNAG domain that comprises only 20 amino acids. The only region that is different between both proteins is the region that separates the zinc finger domains and the SNAG domain. Both proteins are co-expressed in hematopoietic stem cells (HSCs) and, to some extent, in multipotent progenitors (MPPs), but expression is specified as soon as early progenitors and show signs of lineage bias. While expression of GFI1 is maintained in lymphoid primed multipotent progenitors (LMPPs) that have the potential to differentiate into both myeloid and lymphoid cells, GFI1B expression is no longer detectable in these cells. By contrast, GFI1 expression is lost in megakaryocyte precursors (MKPs) and in megakaryocyte-erythrocyte progenitors (MEPs), which maintain a high level of GFI1B expression. Consequently, GFI1 drives myeloid and lymphoid differentiation and GFI1B drives the development of megakaryocytes, platelets, and erythrocytes. How such complementary cell type- and lineage-specific functions of GFI1 and GFI1B are maintained is still an unresolved question in particular since they share an almost identical structure and very similar biochemical modes of actions. The cell type-specific accessibility of GFI1/1B binding sites may explain the fact that very similar transcription factors can be responsible for very different transcriptional programming. An additional explanation comes from recent data showing that both proteins may have additional non-transcriptional functions. GFI1 interacts with a number of proteins involved in DNA repair and lack of GFI1 renders HSCs highly susceptible to DNA damage-induced death and restricts their proliferation. In contrast, GFI1B binds to proteins of the beta-catenin/Wnt signaling pathway and lack of GFI1B leads to an expansion of HSCs and MKPs, illustrating the different impact that GFI1 or GFI1B has on HSCs. In addition, GFI1 and GFI1B are required for endothelial cells to become the first blood cells during early murine development and are among those transcription factors needed to convert adult endothelial cells or fibroblasts into HSCs. This role of GFI1 and GFI1B bears high significance for the ongoing effort to generate hematopoietic stem and progenitor cells de novo for the autologous treatment of blood disorders such as leukemia and lymphoma.
Keywords: GFI1; GFI1B; hair cells; hematopoietic stem cells; hemogenic epithelium; transdifferentiation.
Copyright © 2020 Beauchemin and Möröy.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

