CG8005 Mediates Transit-Amplifying Spermatogonial Divisions via Oxidative Stress in Drosophila Testes
- PMID: 33193998
- PMCID: PMC7641671
- DOI: 10.1155/2020/2846727
CG8005 Mediates Transit-Amplifying Spermatogonial Divisions via Oxidative Stress in Drosophila Testes
Abstract
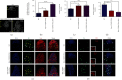
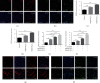
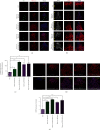
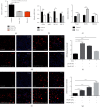
The generation of reactive oxygen species (ROS) widely occurs in metabolic reactions and affects stem cell activity by participating in stem cell self-renewal. However, the mechanisms of transit-amplifying (TA) spermatogonial divisions mediated by oxidative stress are not fully understood. Through genetic manipulation of Drosophila testes, we demonstrated that CG8005 regulated TA spermatogonial divisions and redox homeostasis. Using in vitro approaches, we showed that the knockdown of CG8005 increased ROS levels in S2 cells; the induced ROS generation was inhibited by NAC and exacerbated by H2O2 pretreatments. Furthermore, the silencing of CG8005 increased the mRNA expression of oxidation-promoting factors Keap1, GstD1, and Mal-A6 and decreased the mRNA expression of antioxidant factors cnc, Gclm, maf-S, ND-42, and ND-75. We further investigated the functions of the antioxidant factor cnc, a key factor in the Keap1-cnc signaling pathway, and showed that cnc mimicked the phenotype of CG8005 in both Drosophila testes and S2 cells. Our results indicated that CG8005, together with cnc, controlled TA spermatogonial divisions by regulating oxidative stress in Drosophila.
Copyright © 2020 Wanyin Chen et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
CG6015 controls spermatogonia transit-amplifying divisions by epidermal growth factor receptor signaling in Drosophila testes.Cell Death Dis. 2021 May 14;12(5):491. doi: 10.1038/s41419-021-03783-9. Cell Death Dis. 2021. PMID: 33990549 Free PMC article.
-
Effects of N-acetylcysteine on CG8005 gene-mediated proliferation and apoptosis of Drosophila S2 embryonic cells.Sci Rep. 2023 Aug 2;13(1):12502. doi: 10.1038/s41598-023-39668-4. Sci Rep. 2023. PMID: 37532734 Free PMC article.
-
[Functional analysis of CG8005 gene in Drosophila testis].Yi Chuan. 2020 Nov 20;42(11):1122-1132. doi: 10.16288/j.yczz.20-163. Yi Chuan. 2020. PMID: 33229318 Chinese.
-
Accumulation of a differentiation regulator specifies transit amplifying division number in an adult stem cell lineage.Proc Natl Acad Sci U S A. 2009 Dec 29;106(52):22311-6. doi: 10.1073/pnas.0912454106. Epub 2009 Dec 14. Proc Natl Acad Sci U S A. 2009. PMID: 20018708 Free PMC article.
-
Mechanisms and functions of Nrf2 signaling in Drosophila.Free Radic Biol Med. 2015 Nov;88(Pt B):302-313. doi: 10.1016/j.freeradbiomed.2015.06.020. Epub 2015 Jun 25. Free Radic Biol Med. 2015. PMID: 26117322 Free PMC article. Review.
Cited by
-
The Complex Landscape of Structural Divergence Between the Drosophila pseudoobscura and D. persimilis Genomes.Genome Biol Evol. 2024 Mar 2;16(3):evae047. doi: 10.1093/gbe/evae047. Genome Biol Evol. 2024. PMID: 38482945 Free PMC article.
-
CG6015 controls spermatogonia transit-amplifying divisions by epidermal growth factor receptor signaling in Drosophila testes.Cell Death Dis. 2021 May 14;12(5):491. doi: 10.1038/s41419-021-03783-9. Cell Death Dis. 2021. PMID: 33990549 Free PMC article.
-
Effects of N-acetylcysteine on CG8005 gene-mediated proliferation and apoptosis of Drosophila S2 embryonic cells.Sci Rep. 2023 Aug 2;13(1):12502. doi: 10.1038/s41598-023-39668-4. Sci Rep. 2023. PMID: 37532734 Free PMC article.
-
Drosophila melanogaster Sting mediates Coxiella burnetii infection by reducing accumulation of reactive oxygen species.Infect Immun. 2024 Mar 12;92(3):e0056022. doi: 10.1128/iai.00560-22. Epub 2024 Feb 16. Infect Immun. 2024. PMID: 38363133 Free PMC article.
-
The Potential Reversible Transition between Stem Cells and Transient-Amplifying Cells: The Limbal Epithelial Stem Cell Perspective.Cells. 2024 Apr 25;13(9):748. doi: 10.3390/cells13090748. Cells. 2024. PMID: 38727284 Free PMC article. Review.
References
-
- Carbonell A., Pérez-Montero S., Climent-Cantó P., Reina O., Azorín F. The germline linker histone dBigH1 and the translational regulator bam form a repressor loop essential for male germ stem cell differentiation. Cell Reports. 2017;21(11):3178–3189. doi: 10.1016/j.celrep.2017.11.060. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

