Neuroglial activation in the auditory cortex and medial geniculate body of salicylate-induced tinnitus rats
- PMID: 33194013
- PMCID: PMC7653558
Neuroglial activation in the auditory cortex and medial geniculate body of salicylate-induced tinnitus rats
Abstract
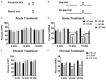
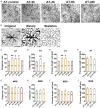
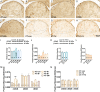
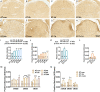
Neuroglial activation has been recognized as a pathological hallmark of a variety of neurological diseases, yet the role of neuroglia in tinnitus hasn't been well established so far. To explore the potential roles of two types of glia cells (astrocyte and microglia) in the development of tinnitus, we examined markers associated with them in the primary auditory (A1) cortex and medial geniculate body (MGB) of rats with salicylate-induced tinnitus. The results demonstrated that acute and chronic administrations of salicylate could cause reversible tinnitus-like behavior in rats. The expression level of GFAP markedly increased in the A1 cortex of rats following acute and chronic treatments of salicylate, accompanied by increased endpoint and process length of astrocyte. The expression level of GFAP and the morphology of astrocyte in the rat MGB remained almost constant following salicylate treatment. On the other hand, the expression level of Iba1 markedly increased in the rat A1 cortex and MGB following acute and chronic treatments of salicylate, together with increased endpoint and process length of microglia in the MGB. Additionally, interleukin 1β (IL-1β), a pro-inflammatory cytokine released by activated glia was significantly up-regulated in the A1 cortex and MGB of rats after salicylate treatments. These findings highlight astrocyte activation and microglia proliferation in the central auditory system of rats experiencing tinnitus, which potently implicate an indispensable glial regulation in tinnitus development.
Keywords: Tinnitus; astrocyte; microglia; neuroglial activation; neuroinflammation; salicylate.
AJTR Copyright © 2020.
Conflict of interest statement
None.
Figures



References
-
- Baizer JS, Lobarinas E, Salvi R, Allman BL. Brain research special issue: advances in the neuroscience of tinnitus. Brain Res. 2012;1485:1–2. - PubMed
-
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. - PubMed
-
- McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70–79. - PubMed
-
- Ryan D, Bauer CA. Neuroscience of tinnitus. Neuroimaging Clin N Am. 2016;26:187–196. - PubMed
-
- Adjamian P, Sereda M, Hall DA. The mechanisms of tinnitus: perspectives from human functional neuroimaging. Hear Res. 2009;253:15–31. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
