Imaging of Non-atherosclerotic Vasculopathies
- PMID: 33194304
- PMCID: PMC7656038
- DOI: 10.25259/JCIS_91_2020
Imaging of Non-atherosclerotic Vasculopathies
Abstract
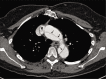
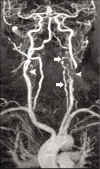
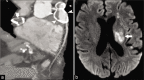
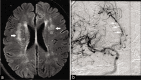
Non-atherosclerotic vasculopathies (NAVs) may present with various neurological symptoms ranging from headache, stroke, visual symptoms, and various types of intracranial hemorrhage. NAVs result from different etiologies which include collagenopathies, immunological, hematological, and infection mechanisms, and other rarer unidentifiable or idiopathic causes. NAV etiologies account for about 10-15% and 20-25% of adult and pediatric stroke cases, respectively, and therefore, diagnosing the underlying cause of NAV becomes clinically very important. Clinical diagnosis of NAV is challenging because the clinical presentation is very non-specific and overlapping with various other central nervous system disorders. Before the advent of non-invasive techniques, making a diagnosis of non-atherosclerotic vasculopathy as a cause of the stroke was very challenging. Today with newer techniques such as high-resolution magnetic resonance (MR), MR and computed tomography perfusion, and angiogram, there are number of pointers which can give us a lead about the non-atherosclerotic causes. Imaging may provide the first lead to the clinician regarding the diagnosis or possible differential diagnosis so that the targeted and focused biomarkers (blood, cerebrospinal fluid, or/and in some cases biopsies) may be obtained to clinch the diagnoses. The purpose of the article is to enumerate the causes, clinical features, and illustrate the imaging findings of the various non-atherosclerotic vasculopathic disorders and discuss "pearls" to their diagnosis. In this article, we have also discussed the latest advances in vascular imaging and elaborated on few uncommon non-atherosclerotic vasculopathies. These are very relevant clinically in the day-to-day practice for the radiologist, neurologist, and the neurointerventionalist.
Keywords: Angiogram; Computed tomography angiography; Magnetic resonance angiography; Vasculopathy.
© 2020 Published by Scientific Scholar on behalf of Journal of Clinical Imaging Science.
Conflict of interest statement
There are no conflicts of interest.
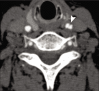
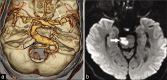
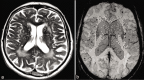
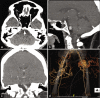
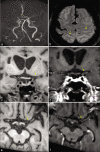
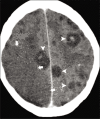
Figures









References
Publication types
LinkOut - more resources
Full Text Sources
