Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers
- PMID: 33194645
- PMCID: PMC7642726
- DOI: 10.3389/fonc.2020.566511
Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers
Abstract
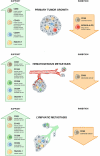
Tumor-associated macrophages (TAMs) are major innate immune cells that constitute up to 50% of the cell mass of human tumors. TAMs are highly heterogeneous cells that originate from resident tissue-specific macrophages and from newly recruited monocytes. TAMs' variability strongly depends on cancer type, stage, and intratumor heterogeneity. Majority of TAMs are programmed by tumor microenvironment to support primary tumor growth and metastatic spread. However, TAMs can also restrict tumor growth and metastasis. In this review, we summarized the knowledge about the role of TAMs in tumor growth, metastasis and in the response to cancer therapy in patients with five aggressive types of cancer: breast, colorectal, lung, ovarian, and prostate cancers that are frequently metastasize into distant organs resulting in high mortality of the patients. Two major TAM parameters are applied for the evaluation of TAM correlation with the cancer progression: total amount of TAMs and specific phenotype of TAMs identified by functional biomarkers. We summarized the data generated in the wide range of international patient cohorts on the correlation of TAMs with clinical and pathological parameters of tumor progression including lymphatic and hematogenous metastasis, recurrence, survival, therapy efficiency. We described currently available biomarkers for TAMs that can be measured in patients' samples (tumor tissue and blood). CD68 is the major biomarker for the quantification of total TAM amounts, while transmembrane receptors (stabilin-1, CD163, CD206, CD204, MARCO) and secreted chitinase-like proteins (YKL-39, YKL-40) are used as biomarkers for the functional TAM polarization. We also considered that specific role of TAMs in tumor progression can depend on the localization in the intratumoral compartments. We have made the conclusion for the role of TAMs in primary tumor growth, metastasis, and therapy sensitivity for breast, colorectal, lung, ovarian, and prostate cancers. In contrast to other cancer types, majority of clinical studies indicate that TAMs in colorectal cancer have protective role for the patient and interfere with primary tumor growth and metastasis. The accumulated data are essential for using TAMs as biomarkers and therapeutic targets to develop cancer-specific immunotherapy and to design efficient combinations of traditional therapy and new immunomodulatory approaches.
Keywords: biomarker; chemotherapy; hematogenous metastasis; immunotherapy; lymphatic metastasis; monocyte, CD68; tumor-associated macrophage.
Copyright © 2020 Larionova, Tuguzbaeva, Ponomaryova, Stakheyeva, Cherdyntseva, Pavlov, Choinzonov and Kzhyshkowska.
Figures

Similar articles
-
Tumor-associated macrophages in human breast cancer parenchyma negatively correlate with lymphatic metastasis after neoadjuvant chemotherapy.Immunobiology. 2017 Jan;222(1):101-109. doi: 10.1016/j.imbio.2016.08.001. Epub 2016 Aug 4. Immunobiology. 2017. PMID: 27510849
-
The intratumoral distribution influences the prognostic impact of CD68- and CD204-positive macrophages in non-small cell lung cancer.Lung Cancer. 2018 Sep;123:127-135. doi: 10.1016/j.lungcan.2018.07.015. Epub 2018 Jul 19. Lung Cancer. 2018. PMID: 30089583
-
CD68+, but not stabilin-1+ tumor associated macrophages in gaps of ductal tumor structures negatively correlate with the lymphatic metastasis in human breast cancer.Immunobiology. 2017 Jan;222(1):31-38. doi: 10.1016/j.imbio.2015.09.011. Epub 2015 Sep 8. Immunobiology. 2017. PMID: 26391151
-
Tumor-Associated Macrophages (TAMs): A Critical Activator In Ovarian Cancer Metastasis.Onco Targets Ther. 2019 Oct 21;12:8687-8699. doi: 10.2147/OTT.S216355. eCollection 2019. Onco Targets Ther. 2019. PMID: 31695427 Free PMC article. Review.
-
The role of tumor-associated macrophage in breast cancer biology.Histol Histopathol. 2018 Feb;33(2):133-145. doi: 10.14670/HH-11-916. Epub 2017 Jul 6. Histol Histopathol. 2018. PMID: 28681373 Review.
Cited by
-
The tumor innate immune microenvironment in prostate cancer: an overview of soluble factors and cellular effectors.Explor Target Antitumor Ther. 2022;3(5):694-718. doi: 10.37349/etat.2022.00108. Epub 2022 Oct 31. Explor Target Antitumor Ther. 2022. PMID: 36338516 Free PMC article. Review.
-
PFKFB3 overexpression in monocytes of patients with colon but not rectal cancer programs pro-tumor macrophages and is indicative for higher risk of tumor relapse.Front Immunol. 2023 Jan 17;13:1080501. doi: 10.3389/fimmu.2022.1080501. eCollection 2022. Front Immunol. 2023. PMID: 36733385 Free PMC article.
-
Activity-Based NIR Bioluminescence Probe Enables Discovery of Diet-Induced Modulation of the Tumor Microenvironment via Nitric Oxide.ACS Cent Sci. 2022 Apr 27;8(4):461-472. doi: 10.1021/acscentsci.1c00317. Epub 2022 Mar 16. ACS Cent Sci. 2022. PMID: 35505872 Free PMC article.
-
Macrophage scavenger receptors: Tumor support and tumor inhibition.Front Oncol. 2023 Jan 6;12:1096897. doi: 10.3389/fonc.2022.1096897. eCollection 2022. Front Oncol. 2023. PMID: 36686729 Free PMC article. Review.
-
Agonistic and antagonistic targeting of immune checkpoint molecules differentially regulate osteoclastogenesis.Front Immunol. 2023 Feb 2;14:988365. doi: 10.3389/fimmu.2023.988365. eCollection 2023. Front Immunol. 2023. PMID: 36817431 Free PMC article.
References
-
- Kzhyshkowska J, Grigoryeva E, Larionova I. Targeting the Tumor-Associated Macrophages for ‘Normalizing’ Cancer. In: Bizzarri M, editor. Approaching Complex Diseases, Human Perspectives in Health Sciences and Technologies 2. Cham: Springer Nature Switzerland AG; (2020). p. 245–74.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

