Overall Survival in Heart Disease-Related Death in Non-Small Cell Lung Cancer Patients: Nonimmunotherapy Versus Immunotherapy Era: Population-Based Study
- PMID: 33194672
- PMCID: PMC7643546
- DOI: 10.3389/fonc.2020.572380
Overall Survival in Heart Disease-Related Death in Non-Small Cell Lung Cancer Patients: Nonimmunotherapy Versus Immunotherapy Era: Population-Based Study
Abstract
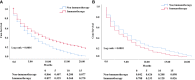
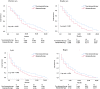
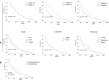
The cardiotoxicity during immunotherapy administration leads to mortality by more than 42% and heart disease-related mortality among immunotherapy-linked cancers is still considered to be underestimated. In this study, the advanced stage of non-small cell lung cancer (NSCLC) with heart disease-related death was selected in accordance with immunotherapy approval time. NSCLC was searched on the Surveillance, Epidemiology, and End Results (SEER) program. Results show that 538 advanced NSCLC cases, those dominated by men and elderly people aged more than 70 years, had a high percentage of heart disease-related death in both eras. The difference between contemporary groups was fairly nonsignificant (P = > 0.05). The overall survival (OS) of all-cause mortality difference showed improved survival in the immunotherapy group (P = 0.0001). In the study of heart disease-related death survival with adjusted data, the NSCLC patients show significant lower survival in the immunotherapy era compared with the nonimmunotherapy era (P = 0.003; hazard ratio [HR] = 1.31; 95% CI = 1.099-1.57). In the multivariate analysis of NSCLC-related immunotherapy, histology revealed that the non-squamous cell type had an independent risk for lower OS than the squamous cell type (P = 0.04; HR= 0.74; CI = 0, 55- 0.99). The results demonstrate the survival benefits for NSCLC in immunotherapy; however, in heart disease-related death, immunotherapy in patients with NSCLC shows decreased OS. This study highlights that NSCLC patients should be highly monitored during immunotherapy administration, and further assessment is needed.
Keywords: SEER database; cardiotoxicity; heart diseases; immunotherapy; non-small cell lung cancer.
Copyright © 2020 Safi, Kanesvaran, Alradhi, Al-Danakh, Ping, Al-Sabai, Shan and Liu.
Figures
Comment in
-
Commentary: Overall Survival in Heart Disease-Related Death in Non-Small Cell Lung Cancer Patients: Nonimmunotherapy Versus Immunotherapy Era: Population-Based Study.Front Oncol. 2021 Apr 22;11:639042. doi: 10.3389/fonc.2021.639042. eCollection 2021. Front Oncol. 2021. PMID: 33968737 Free PMC article. No abstract available.
References
-
- Salem J-E, Manouchehri A, Moey M, Lebrun-Vignes B., Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol (2018) 19(12):1579–89. 10.1016/S1470-2045(18)30608-9 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

