Endocytic Rabs Are Recruited to the Trypanosoma cruzi Parasitophorous Vacuole and Contribute to the Process of Infection in Non-professional Phagocytic Cells
- PMID: 33194787
- PMCID: PMC7658340
- DOI: 10.3389/fcimb.2020.536985
Endocytic Rabs Are Recruited to the Trypanosoma cruzi Parasitophorous Vacuole and Contribute to the Process of Infection in Non-professional Phagocytic Cells
Abstract
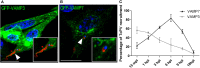
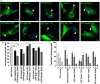
Trypanosoma cruzi is the parasite causative of Chagas disease, a highly disseminated illness endemic in Latin-American countries. T. cruzi has a complex life cycle that involves mammalian hosts and insect vectors both of which exhibits different parasitic forms. Trypomastigotes are the infective forms capable to invade several types of host cells from mammals. T. cruzi infection process comprises two sequential steps, the formation and the maturation of the Trypanosoma cruzi parasitophorous vacuole. Host Rab GTPases are proteins that control the intracellular vesicular traffic by regulating budding, transport, docking, and tethering of vesicles. From over 70 Rab GTPases identified in mammalian cells only two, Rab5 and Rab7 have been found in the T. cruzi vacuole to date. In this work, we have characterized the role of the endocytic, recycling, and secretory routes in the T. cruzi infection process in CHO cells, by studying the most representative Rabs of these pathways. We found that endocytic Rabs are selectively recruited to the vacuole of T. cruzi, among them Rab22a, Rab5, and Rab21 right away after the infection followed by Rab7 and Rab39a at later times. However, neither recycling nor secretory Rabs were present in the vacuole membrane at the times studied. Interestingly loss of function of endocytic Rabs by the use of their dominant-negative mutant forms significantly decreases T. cruzi infection. These data highlight the contribution of these proteins and the endosomal route in the process of T. cruzi infection.
Keywords: Rab proteins; T. cruzi parasitophorous vacuole; Trypanosoma cruzi; endocytosis; host cell infection.
Copyright © 2020 Salassa, Cueto, Gambarte Tudela and Romano.
Figures

Similar articles
-
The host Rab9a/Rab32 axis is actively recruited to the Trypanosoma cruzi parasitophorous vacuole and benefits the infection cycle.Mol Microbiol. 2024 Nov;122(5):643-659. doi: 10.1111/mmi.15217. Epub 2024 Jan 9. Mol Microbiol. 2024. PMID: 38193389
-
Soluble N-ethylmaleimide-sensitive factor attachment protein receptors required during Trypanosoma cruzi parasitophorous vacuole development.Cell Microbiol. 2017 Jun;19(6). doi: 10.1111/cmi.12713. Epub 2017 Jan 17. Cell Microbiol. 2017. PMID: 27992096
-
Novel PI 3-kinase-dependent mechanisms of trypanosome invasion and vacuole maturation.J Cell Sci. 2003 Sep 1;116(Pt 17):3611-22. doi: 10.1242/jcs.00666. Epub 2003 Jul 22. J Cell Sci. 2003. PMID: 12876217
-
Mechanisms of host cell invasion by Trypanosoma cruzi.Adv Parasitol. 2011;76:33-61. doi: 10.1016/B978-0-12-385895-5.00002-5. Adv Parasitol. 2011. PMID: 21884886 Review.
-
Molecular and cellular mechanisms involved in the Trypanosoma cruzi/host cell interplay.IUBMB Life. 2012 May;64(5):387-96. doi: 10.1002/iub.1019. Epub 2012 Mar 27. IUBMB Life. 2012. PMID: 22454195 Free PMC article. Review.
Cited by
-
Autophagy in protists and their hosts: When, how and why?Autophagy Rep. 2023;2(1):2149211. doi: 10.1080/27694127.2022.2149211. Epub 2023 Mar 9. Autophagy Rep. 2023. PMID: 37064813 Free PMC article.
-
All Roads Lead to Cytosol: Trypanosoma cruzi Multi-Strategic Approach to Invasion.Front Cell Infect Microbiol. 2021 Mar 5;11:634793. doi: 10.3389/fcimb.2021.634793. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33747982 Free PMC article. Review.
-
Immunopathological Mechanisms Underlying Cardiac Damage in Chagas Disease.Pathogens. 2023 Feb 16;12(2):335. doi: 10.3390/pathogens12020335. Pathogens. 2023. PMID: 36839607 Free PMC article. Review.
-
Trypanosoma cruzi has Two Peptidyl-tRNA Hydrolases Showing Different Localization and Function.Acta Parasitol. 2025 Feb 13;70(1):60. doi: 10.1007/s11686-025-00989-1. Acta Parasitol. 2025. PMID: 39945942
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

