Caprine Bactenecins as Promising Tools for Developing New Antimicrobial and Antitumor Drugs
- PMID: 33194795
- PMCID: PMC7604311
- DOI: 10.3389/fcimb.2020.552905
Caprine Bactenecins as Promising Tools for Developing New Antimicrobial and Antitumor Drugs
Abstract
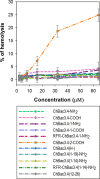
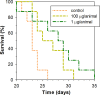
Proline-rich antimicrobial peptides (PR-AMPs) having a potent antimicrobial activity predominantly toward Gram-negative bacteria and negligible toxicity toward host cells, are attracting attention as new templates for developing antibiotic drugs. We have previously isolated and characterized several bactenecins that are promising in this respect, from the leukocytes of the domestic goat Capra hircus: ChBac5, miniChBac7.5N-α, and -β, as well as ChBac3.4. Unlike the others, ChBac3.4 shows a somewhat unusual pattern of activities for a mammalian PR-AMP: it is more active against bacterial membranes as well as tumor and, to the lesser extent, normal cells. Here we describe a SAR study of ChBac3.4 (RFRLPFRRPPIRIHPPPFYPPFRRFL-NH2) which elucidates its peculiarities and evaluates its potential as a lead for antimicrobial or anticancer drugs based on this peptide. A set of designed structural analogues of ChBac3.4 was explored for antibacterial activity toward drug-resistant clinical isolates and antitumor properties. The N-terminal region was found to be important for the antimicrobial action, but not responsible for the toxicity toward mammalian cells. A shortened variant with the best selectivity index toward bacteria demonstrated a pronounced synergy in combination with antibiotics against Gram-negative strains, albeit with a somewhat reduced ability to inhibit biofilm formation compared to native peptide. C-terminal amidation was examined for some analogues, which did not affect antimicrobial activity, but somewhat altered the cytotoxicity toward host cells. Interestingly, non-amidated peptides showed a slight delay in their impact on bacterial membrane integrity. Peptides with enhanced hydrophobicity showed increased toxicity, but in most cases their selectivity toward tumor cells also improved. While most analogues lacked hemolytic properties, a ChBac3.4 variant with two additional tryptophan residues demonstrated an appreciable activity toward human erythrocytes. The variant demonstrating the best tumor/nontumor cell selectivity was found to more actively initiate apoptosis in target cells, though its action was slower than that of the native ChBac3.4. Its antitumor effectiveness was successfully verified in vivo in a murine Ehrlich ascites carcinoma model. The obtained results demonstrate the potential of structural modification to manage caprine bactenecins' selectivity and activity spectrum and confirm that they are promising prototypes for antimicrobial and anticancer drugs design.
Keywords: antibacterial activity; antibiofilm activity; antibiotics; antitumor activity; bactenecins; proline-rich antimicrobial peptides; structure–activity relationship; synergy.
Copyright © 2020 Kopeikin, Zharkova, Kolobov, Smirnova, Sukhareva, Umnyakova, Kokryakov, Orlov, Milman, Balandin, Panteleev, Ovchinnikova, Komlev, Tossi and Shamova.
Figures



References
-
- Agerberth B., Lee J. Y., Bergman T., Carlquist M., Boman H. G., Mutt V., et al. (1991). Amino acid sequence of PR-39. Isolation from pig intestine of a new member of the family of proline-arginine-rich antibacterial peptides. Eur. J. Biochem. 202, 849–854. 10.1111/j.1432-1033.1991.tb16442.x - DOI - PubMed
-
- Balandin S. V., Emelianova A. A., Kalashnikova M. B., Kokryakov V. N., Shamova O. V., Ovchinnikova T. V. (2016). Molecular mechanisms of antitumor effect of natural antimicrobial peptides. Russ. J. Bioorg. Chem. 42 (6), 575–589. 10.1134/S1068162016060029 - DOI
-
- Bulet P. (2008). “Strategies for the Discovery, Isolation, and Characterization of Natural Bioactive Peptides from the Immune System of Invertebrates,” in Peptide-Based Drug Design. Methods In Molecular Biology™, vol. 494 Ed. Otvos L. (New York, NY: Humana Press; ), 9–29. 10.1007/978-1-59745-419-3_2 - DOI - PubMed
-
- Casciaro B., Lin Q., Afonin S., Loffredo M. R., de Turris V., Middel V., et al. (2019). Inhibition of Pseudomonas aeruginosa biofilm formation and expression of virulence genes by selective epimerization in the peptide Esculentin-1a(1-21)NH2. FEBS J. 286 (19), 3874–3891. 10.1111/febs.14940 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

