Low Density Lipoprotein Receptor-Related Protein-1 (LRP1) Is Involved in the Uptake of Clostridioides difficile Toxin A and Serves as an Internalizing Receptor
- PMID: 33194803
- PMCID: PMC7604483
- DOI: 10.3389/fcimb.2020.565465
Low Density Lipoprotein Receptor-Related Protein-1 (LRP1) Is Involved in the Uptake of Clostridioides difficile Toxin A and Serves as an Internalizing Receptor
Abstract
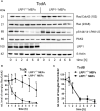
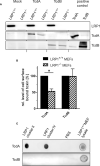
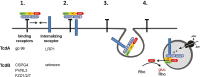
Toxin producing Clostridioides difficile strains cause gastrointestinal infections with the large glucosylating protein toxins A (TcdA) and B (TcdB) being major virulence factors responsible for the onset of symptoms. TcdA and TcdB enter their target cells via receptor-mediated endocytosis. Inside the cell, the toxins glucosylate and thereby inactivate small GTPases of the Rho-/Ras subfamilies resulting in actin reorganization and cell death. The receptors of TcdA are still elusive, glycoprotein 96 (gp96), the low density lipoprotein receptor family (LDLR) and sulfated glycosaminoglycans (sGAGs) have most recently been suggested as receptors for TcdA. In this study, we provide evidence on rapid endocytosis of Low density lipoprotein Receptor-related Protein-1 (LRP1) into fibroblasts and Caco-2 cells by exploiting biotinylation of cell surface proteins. In contrast, gp96 was not endocytosed either in the presence or absence of TcdA. The kinetics of internalization of TfR and LRP1 were comparable in the presence and the absence of TcdA, excluding that TcdA facilitates its internalization by triggering internalization of its receptors. Exploiting fibroblasts with a genetic deletion of LRP1, TcdA was about one order of magnitude less potent in LRP1-deficient cells as compared to the corresponding control cells. In contrast, TcdB exhibited a comparable potency in LRP1-proficient and -deficient fibroblasts. These findings suggested a role of LRP1 in the cellular uptake of TcdA but not of TcdB. Correspondingly, binding of TcdA to the cell surface of LRP1-deficient fibroblasts was reduced as compared with LRP1-proficient fibroblasts. Finally, TcdA bound to LRP1 ligand binding type repeat cluster II (amino acid 786-1,165) and cluster IV (amino acid 3332-3779). In conclusion, LRP1 appears to serve as an endocytic receptor and gp96 as a non-endocytic receptor for TcdA.
Keywords: cell surface; clostridial glycosylating toxins; clostridioides difficile infection; endocytosis; receptors.
Copyright © 2020 Schöttelndreier, Langejürgen, Lindner and Genth.
Figures


Similar articles
-
Expression and (Lacking) Internalization of the Cell Surface Receptors of Clostridioides difficile Toxin B.Front Microbiol. 2018 Jul 4;9:1483. doi: 10.3389/fmicb.2018.01483. eCollection 2018. Front Microbiol. 2018. PMID: 30022975 Free PMC article.
-
The antimicrobial peptide Angie 5 inhibits TcdA and TcdB from Clostridioides difficile.Cell Mol Life Sci. 2025 Jun 30;82(1):265. doi: 10.1007/s00018-025-05799-2. Cell Mol Life Sci. 2025. PMID: 40586877 Free PMC article.
-
Inhibition of Clostridioides difficile toxins TcdA and TcdB by the amiodarone derivative dronedarone.Naunyn Schmiedebergs Arch Pharmacol. 2024 Dec;397(12):9877-9885. doi: 10.1007/s00210-024-03248-8. Epub 2024 Jun 27. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38935126 Free PMC article.
-
Cytotoxic synergism of Clostridioides difficile toxin B with proinflammatory cytokines in subjects with inflammatory bowel diseases.World J Gastroenterol. 2023 Jan 28;29(4):582-596. doi: 10.3748/wjg.v29.i4.582. World J Gastroenterol. 2023. PMID: 36742168 Free PMC article. Review.
-
Clostridium difficile toxins: more than mere inhibitors of Rho proteins.Int J Biochem Cell Biol. 2008;40(4):592-7. doi: 10.1016/j.biocel.2007.12.014. Epub 2008 Jan 5. Int J Biochem Cell Biol. 2008. PMID: 18289919 Review.
Cited by
-
LDL receptor-mediated endocytosis of Escherichia coli α-hemolysin mediates renal epithelial toxicity.Proc Natl Acad Sci U S A. 2025 Jun 17;122(24):e2505482122. doi: 10.1073/pnas.2505482122. Epub 2025 Jun 12. Proc Natl Acad Sci U S A. 2025. PMID: 40504153
-
Identification of TFPI as a receptor reveals recombination-driven receptor switching in Clostridioides difficile toxin B variants.Nat Commun. 2022 Nov 9;13(1):6786. doi: 10.1038/s41467-022-33964-9. Nat Commun. 2022. PMID: 36351897 Free PMC article.
-
Clostridioides difficile toxins: mechanisms of action and antitoxin therapeutics.Nat Rev Microbiol. 2022 May;20(5):285-298. doi: 10.1038/s41579-021-00660-2. Epub 2021 Nov 26. Nat Rev Microbiol. 2022. PMID: 34837014 Free PMC article. Review.
-
Molecular basis of TMPRSS2 recognition by Paeniclostridium sordellii hemorrhagic toxin.Nat Commun. 2024 Mar 4;15(1):1976. doi: 10.1038/s41467-024-46394-6. Nat Commun. 2024. PMID: 38438396 Free PMC article.
-
Structural dynamics of the CROPs domain control stability and toxicity of Paeniclostridium sordellii lethal toxin.Nat Commun. 2023 Dec 19;14(1):8426. doi: 10.1038/s41467-023-44169-z. Nat Commun. 2023. PMID: 38114525 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

