Implications of TORCH Diseases in Retinal Development-Special Focus on Congenital Toxoplasmosis
- PMID: 33194824
- PMCID: PMC7649341
- DOI: 10.3389/fcimb.2020.585727
Implications of TORCH Diseases in Retinal Development-Special Focus on Congenital Toxoplasmosis
Abstract
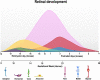
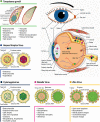
There are certain critical periods during pregnancy when the fetus is at high risk for exposure to teratogens. Some microorganisms, including Toxoplasma gondii, are known to exhibit teratogenic effects, interfering with fetal development and causing irreversible disturbances. T. gondii is an obligate intracellular parasite and the etiological agent of Toxoplasmosis, a zoonosis that affects one third of the world's population. Although congenital infection can cause severe fetal damage, the injury extension depends on the gestational period of infection, among other factors, like parasite genotype and host immunity. This parasite invades the Central Nervous System (CNS), forming tissue cysts, and can interfere with neurodevelopment, leading to frequent neurological abnormalities associated with T. gondii infection. Therefore, T. gondii is included in the TORCH complex of infectious diseases that may lead to neurological malformations (Toxoplasmosis, Others, Rubella, Cytomegalovirus, and Herpes). The retina is part of CNS, as it is derived from the diencephalon. Except for astrocytes and microglia, retinal cells originate from multipotent neural progenitors. After cell cycle exit, cells migrate to specific layers, undergo morphological and neurochemical differentiation, form synapses and establish their circuits. The retina is organized in nuclear layers intercalated by plexus, responsible for translating and preprocessing light stimuli and for sending this information to the brain visual nuclei for image perception. Ocular toxoplasmosis (OT) is a very debilitating condition and may present high severity in areas in which virulent strains are found. However, little is known about the effect of congenital infection on the biology of retinal progenitors/ immature cells and how this infection may affect the development of this tissue. In this context, this study reviews the effects that congenital infections may cause to the developing retina and the cellular and molecular aspects of these diseases, with special focus on congenital OT.
Keywords: TORCH; Toxoplasma gondii; congenital infections; congenital toxoplasmosis; retinal development; teratogenesis.
Copyright © 2020 Campos, Calaza and Adesse.
Figures

References
-
- Ambroise-Thomas P., Petersen E. (2000). Congenital toxoplasmosis: past, present and future, in Congenital Toxoplasmosis, eds. Ambroise-Thomas P., Petersen E. (Berlin: Springer-Verlag France; ), 1–7. 10.1093/jpids/piu077 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

