Host Cell Targets of Released Lipid and Secreted Protein Effectors of Mycobacterium tuberculosis
- PMID: 33194845
- PMCID: PMC7644814
- DOI: 10.3389/fcimb.2020.595029
Host Cell Targets of Released Lipid and Secreted Protein Effectors of Mycobacterium tuberculosis
Abstract
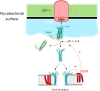
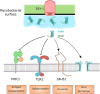
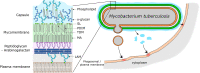
Mycobacterium tuberculosis (Mtb) is a very successful pathogen, strictly adapted to humans and the cause of tuberculosis. Its success is associated with its ability to inhibit host cell intrinsic immune responses by using an arsenal of virulence factors of different nature. It has evolved to synthesize a series of complex lipids which form an outer membrane and may also be released to enter host cell membranes. In addition, secreted protein effectors of Mtb are entering the host cell cytosol to interact with host cell proteins. We briefly discuss the current model, involving the ESX-1 type seven secretion system and the Mtb lipid phthiocerol dimycoserosate (PDIM), of how Mtb creates pores in the phagosomal membrane to allow Mtb proteins to access to the host cell cytosol. We provide an exhaustive list of Mtb secreted proteins that have effector functions. They modify (mostly inhibit but sometimes activate) host cell pathways such as: phagosome maturation, cell death, cytokine response, xenophagy, reactive oxygen species (ROS) response via NADPH oxidase 2 (NOX2), nitric oxide (NO) response via NO Synthase 2 (NOS2) and antigen presentation via MHC class I and class II molecules. We discuss the host cell targets for each lipid and protein effector and the importance of the Mtb effector for virulence of the bacterium.
Keywords: ESX-1; Mycobacterium tuberculosis; NOX2; cell death; cytokines; effector; lipids; phagosome maturation.
Copyright © 2020 Augenstreich and Briken.
Figures
References
-
- Antonelli L. R. V., Gigliotti Rothfuchs A., Gonçalves R., Roffê E., Cheever A. W., Bafica A., et al. (2010). Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J. Clin. Invest. 120, 1674–1682. 10.1172/JCI40817 - DOI - PMC - PubMed
-
- Arbues A., Lugo-Villarino G., Neyrolles O., Guilhot C., Astarie-Dequeker C. (2014). Playing hide-and-seek with host macrophages through the use of mycobacterial cell envelope phthiocerol dimycocerosates and phenolic glycolipids. Front. Cell. Infect. Microbiol. 4:173. 10.3389/fcimb.2014.00173 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

