Overgrowth Syndromes-Evaluation, Diagnosis, and Management
- PMID: 33194904
- PMCID: PMC7661798
- DOI: 10.3389/fped.2020.574857
Overgrowth Syndromes-Evaluation, Diagnosis, and Management
Erratum in
-
Corrigendum: Overgrowth Syndromes-Evaluation, Diagnosis, and Management.Front Pediatr. 2020 Dec 23;8:624141. doi: 10.3389/fped.2020.624141. eCollection 2020. Front Pediatr. 2020. PMID: 33425822 Free PMC article.
Abstract
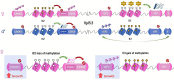
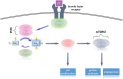
Abnormally excessive growth results from perturbation of a complex interplay of genetic, epigenetic, and hormonal factors that orchestrate human growth. Overgrowth syndromes generally present with inherent health concerns and, in some instances, an increased risk of tumor predisposition that necessitate prompt diagnosis and appropriate referral. In this review, we introduce some of the more common overgrowth syndromes, along with their molecular mechanisms, diagnostics, and medical complications for improved recognition and management of patients affected with these disorders.
Keywords: Beckwith-Wiedemann; PIK3CA; Proteus Syndrome; Pten; Simpson-Golabi-Behmel; Sotos; Weaver; overgrowth.
Copyright © 2020 Manor and Lalani.
Figures


References
-
- Nichols J. Normal Growth Patterns in Infants and Prepubertal Children. Waltham, MA: UpToDate Inc. (2018). Available online at: http://www.uptodate.com (accessed March 2020).
-
- Richmond EJ, Rogol AD. The Child With Tall Stature and/or Abnormally Rapid Growth. (2018). Retrieved from http://www.uptodate.com (accessed April 2020).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

