Porphyrins Through the Looking Glass: Spectroscopic and Mechanistic Insights in Supramolecular Chirogenesis of New Self-Assembled Porphyrin Derivatives
- PMID: 33195087
- PMCID: PMC7593786
- DOI: 10.3389/fchem.2020.587842
Porphyrins Through the Looking Glass: Spectroscopic and Mechanistic Insights in Supramolecular Chirogenesis of New Self-Assembled Porphyrin Derivatives
Abstract
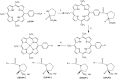
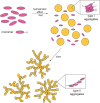
The solvent driven aggregation of porphyrin derivatives, covalently linked to a L- or D-prolinate enantiomer, results in the stereospecific formation of species featuring remarkable supramolecular chirality, as a consequence of reading and amplification of the stereochemical information stored in the proline-appended group. Spectroscopic, kinetic, and topographic SEM studies gave important information on the aggregation processes, and on the structures of the final chiral architectures. The results obtained may be the seeds for the construction of stereoselective sensors aiming at the detection, for example, of novel emergent pollutants from agrochemical, food, and pharmaceutical industry.
Keywords: chirality; circular dichroism; porphyrins; self-assembly; supramolecular chemistry; supramolecular chirogenesis.
Copyright © 2020 Stefanelli, Savioli, Zurlo, Magna, Belviso, Marsico, Superchi, Venanzi, Di Natale, Paolesse and Monti.
Figures





Similar articles
-
Stereospecific Self-Assembly Processes of Porphyrin-Proline Conjugates: From the Effect of Structural Features and Bulk Solvent Properties to the Application in Stereoselective Sensor Systems.Int J Mol Sci. 2022 Dec 9;23(24):15587. doi: 10.3390/ijms232415587. Int J Mol Sci. 2022. PMID: 36555226 Free PMC article. Review.
-
Seeding Chiral Ensembles of Prolinated Porphyrin Derivatives on Glass Surface: Simple and Rapid Access to Chiral Porphyrin Films.Front Chem. 2022 Jan 31;9:804893. doi: 10.3389/fchem.2021.804893. eCollection 2021. Front Chem. 2022. PMID: 35174141 Free PMC article.
-
Tunable Supramolecular Chirogenesis in the Self-Assembling of Amphiphilic Porphyrin Triggered by Chiral Amines.Int J Mol Sci. 2020 Nov 13;21(22):8557. doi: 10.3390/ijms21228557. Int J Mol Sci. 2020. PMID: 33202819 Free PMC article.
-
Kinetic and spectroscopic studies on the chiral self-aggregation of amphiphilic zinc and copper (l)-prolinate-tetraarylporphyrin derivatives in different aqueous media.Org Biomol Chem. 2019 Jan 31;17(5):1113-1120. doi: 10.1039/c8ob02689k. Org Biomol Chem. 2019. PMID: 30633293
-
The Self-Aggregation of Porphyrins with Multiple Chiral Centers in Organic/Aqueous Media: The Case of Sugar- and Steroid-Porphyrin Conjugates.Molecules. 2020 Oct 4;25(19):4544. doi: 10.3390/molecules25194544. Molecules. 2020. PMID: 33020381 Free PMC article. Review.
Cited by
-
Chiral porphyrin-SiO2 nano helices-based sensors for vapor enantiomers recognition.Nanoscale Adv. 2024 Jul 19;6(17):4470-4478. doi: 10.1039/d4na00217b. eCollection 2024 Aug 20. Nanoscale Adv. 2024. PMID: 39170970 Free PMC article.
-
Stereospecific Self-Assembly Processes of Porphyrin-Proline Conjugates: From the Effect of Structural Features and Bulk Solvent Properties to the Application in Stereoselective Sensor Systems.Int J Mol Sci. 2022 Dec 9;23(24):15587. doi: 10.3390/ijms232415587. Int J Mol Sci. 2022. PMID: 36555226 Free PMC article. Review.
-
Seeding Chiral Ensembles of Prolinated Porphyrin Derivatives on Glass Surface: Simple and Rapid Access to Chiral Porphyrin Films.Front Chem. 2022 Jan 31;9:804893. doi: 10.3389/fchem.2021.804893. eCollection 2021. Front Chem. 2022. PMID: 35174141 Free PMC article.
-
Tunable Supramolecular Chirogenesis in the Self-Assembling of Amphiphilic Porphyrin Triggered by Chiral Amines.Int J Mol Sci. 2020 Nov 13;21(22):8557. doi: 10.3390/ijms21228557. Int J Mol Sci. 2020. PMID: 33202819 Free PMC article.
References
-
- Arteaga O., El-Hachemi Z., Canillas A., Crusats J., Rovira M., Ribó J. M. (2016). Reversible and irreversible emergence of chiroptical signals in J-aggregates of achiral 4-sulfonatophenyl substituted porphyrins: intrinsic chirality vs. chiral ordering in the solution. Chem. Commun. 52, 10874–10877. 10.1039/C6CC05709H - DOI - PubMed
-
- Belviso S., Capasso S., Santoro E., Najafi L., Lelj F., Superchi S., et al. (2018a). Thioethyl-porphyrazine/nanocarbon hybrids for photoinduced electron transfer. Adv. Funct. Mater. 28, 1705418 10.1002/adfm.201705418 - DOI
-
- Belviso S., Santoro E., Lelj F., Casarini D., Villani C., Franzini R., et al. (2018b). Stereochemical stability and absolute configuration of atropisomeric thioalkyl-porphyrazines by dynamic NMR and HPLC studies and computational analysis of HPLC-ECD recorded spectra. Eur. J. Org. Chem. 4029–4037. 10.1002/ejoc.201800553 - DOI
-
- Berova N., Nakanishi K., Woody R. W. (2000). Circular Dichroism: Principles and Applications, 2nd Edn. Weinheim: Wiley-WCH.
LinkOut - more resources
Full Text Sources

