Advances in CMV Management: A Single Center Real-Life Experience
- PMID: 33195184
- PMCID: PMC7652755
- DOI: 10.3389/fcell.2020.534268
Advances in CMV Management: A Single Center Real-Life Experience
Abstract
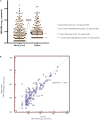
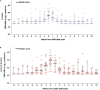
CMV infection is a major challenge in allogeneic stem cell transplantation (allo-SCT). The changing landscape in CMV management includes the introduction of letermovir in prophylaxis of high-risk patients and the source of CMV DNA monitoring (plasma-PL vs. whole blood-WB), for pre-emptive therapy (PET) initiation. We report here how our real-life experience in CMV management evolved, following letermovir registration. We focus on: (i) the effects of systematic use of letermovir for CMV prophylaxis in high-risk patients, (ii) the results of a longitudinal comparison of CMV DNAemia monitoring in PL and WB. From December 2018 to April 2020, 60 allo-SCTs have been performed in our center (LET ERA), of whom 45 received letermovir in prophylaxis from day 0 to day + 100, because of recipient positivity of anti CMV IgG. These patients were compared with a cohort of 41 allo-SCTs performed between November 2017 and November 2018 (NO LET ERA). Firstly, the incidence of CMV clinically significant infections, CMV disease, bacterial infections, proven/probable fungal infections, hospital re-admissions after allo-SCT by day + 100 in the two ERA were 8 vs. 44% (p = 0.0006), 2 vs. 12% (p = 0.02), 37 vs. 56% (p = 0.05), 8 vs. 19% (p = 0.09), and 23 vs. 39% (p = 0.09), respectively. By day + 180 these differences were 17 vs. 68% (p < 0.00001), 2 vs. 12% (p = 0.02), 45 vs. 78% (p = 0.09), 8 vs. 22% (p = 0.05), and 40 vs. 66% (p = 0.01), respectively. Secondly, from February to May 2019, we comparatively measured CMV DNA from WB and PL and we confirmed that there is a linear correlation between CMV DNA level in WB and PL (Spearman's test r = 0.86). Moreover, CMV DNAemia at the time of PET in the 12 patients with a clinically significant CMV infection was higher in WB vs. PL (5.202 vs. 4.981 copies/ml, p = 0.1). Our real-life experience confirms that: (i) letermovir is highly effective, leading to a significant drop in CMV clinically significant infections and CMV-related complications by day + 100 and + 180 after allo-SCT; (ii) WB may be an effective alternative to PL as a source for CMV DNA monitoring, as a linear correlation of DNAemia was confirmed between WB and PL, even if the CMV DNAemia at PET initiation was comparable in the two sources.
Keywords: CMV; CMV DNA monitoring; allogeneic stem cell transplantation; pre-emptive therapy; prophylaxis.
Copyright © 2020 Malagola, Pollara, Polverelli, Zollner, Bettoni, Gandolfi, Gramegna, Morello, Turra, Corbellini, Signorini, Moioli, Bernardi, Zanaglio, Farina, Testa, Caruso and Russo.
Figures
References
-
- Anderson A., Raja M., Vaquez N., Morris M., Komanduri K., Camargo J. (2020). Clinical “real-world” experience with letermovir for prevention of cytomegalovirus infection in allogeneic hematopoietic cell transplantation recipients. Clin. Transplant. 3:e13866. - PubMed
-
- Ariza-Heredia E. J., Nesher L., Chemaly R. F. (2014). Cytomegalovirus disease after stem cell transplantation: a mini-review. Cancer Lett. 342 1–8. - PubMed
-
- Boeckh M., Nichols W. G. (2004). The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 103 2003–2008. - PubMed
-
- Cariani E., Pollara C., Valloncini B., Perandin F., Bonfanti C., Manca N. (2007). Relationship between pp65 antigenemia levels and real-time quantitative DNA PCR for human cytomegalovirus (HCMV) management in immunocompromised patients. BMC Infect. Dis. 7:138. 10.1186/1471-2334-7-138 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

