Initial WNT/β-Catenin Activation Enhanced Mesoderm Commitment, Extracellular Matrix Expression, Cell Aggregation and Cartilage Tissue Yield From Induced Pluripotent Stem Cells
- PMID: 33195222
- PMCID: PMC7661475
- DOI: 10.3389/fcell.2020.581331
Initial WNT/β-Catenin Activation Enhanced Mesoderm Commitment, Extracellular Matrix Expression, Cell Aggregation and Cartilage Tissue Yield From Induced Pluripotent Stem Cells
Abstract
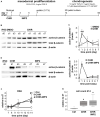
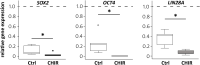
Mesodermal differentiation of induced pluripotent stem cells (iPSCs) in vitro and subsequent specification into mesodermal derivatives like chondrocytes is currently afflicted with a substantial cell loss that severely limits tissue yield. More knowledge on the key players regulating mesodermal differentiation of iPSCs is currently needed to drive all cells into the desired lineage and to overcome the current need for intermediate cell selection steps to remove misdifferentiated cells. Using two independent human iPSC lines, we here report that a short initial WNT/β-catenin pulse induced by the small molecule CHIR99021 (24 h) enhanced expression of mesodermal markers (PDGFRα, HAND1, KDR, and GATA4), supported the exit from pluripotency (decreased OCT4, SOX2, and LIN28A) and inhibited ectodermal misdifferentiation (reduced PAX6, TUBB3, and NES). Importantly, the initial CHIR pulse increased cell proliferation until day 14 (five-fold), adjusted expression of adhesion-related genes (CDH3 up, CDH6 down) and increased extracellular matrix (ECM)-related gene expression (COL6, COL1, COL3, COL5, DCN, NPNT, LUM, MGP, MATN2, and VTN), thus yielding more matrix-interacting progenitors with a high aggregation capability. Enhanced contribution to chondrogenic pellet formation increased the cell yield after eight weeks 200-fold compared to controls. The collagen type II and proteoglycan-positive area was enlarged in the CHIR group, indicating an increased number of cartilage-forming cells. Conclusively, short initial WNT activation improved mesoderm commitment and our data demonstrated for the first time to our knowledge that, acting via stimulation of cell proliferation, ECM expression and cell aggregation, WNT pulsing is a key step to make cell selection steps before chondrogenesis obsolete. This advanced understanding of the WNT/β-catenin function is a major step toward robust and efficient generation of high-quality mesodermal progenitors from human iPSCs and toward rescuing low tissue yield during subsequent in vitro chondrogenesis, which is highly desired for clinical cartilage regeneration, disease modeling and drug screening.
Keywords: WNT/β-catenin; aggregation; cartilage; cell survival; extracellular matrix; induced pluripotent stem cells.
Copyright © 2020 Kreuser, Buchert, Haase, Richter and Diederichs.
Figures




References
-
- Brandenberger R., Schmidt A., Linton J., Wang D., Backus C., Denda S., et al. (2001). Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J. Cell Biol. 154 447–458. 10.1083/jcb.200103069 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

