Disease Phenotypes and Mechanisms of iPSC-Derived Cardiomyocytes From Brugada Syndrome Patients With a Loss-of-Function SCN5A Mutation
- PMID: 33195263
- PMCID: PMC7642519
- DOI: 10.3389/fcell.2020.592893
Disease Phenotypes and Mechanisms of iPSC-Derived Cardiomyocytes From Brugada Syndrome Patients With a Loss-of-Function SCN5A Mutation
Abstract
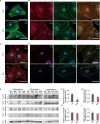
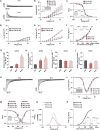
Brugada syndrome (BrS) is one of the major causes of sudden cardiac death in young people, while the underlying mechanisms are not completely understood. Here, we investigated the pathophysiological phenotypes and mechanisms using induced pluripotent stem cell (iPSC)-derived cardiomyocytes (CMs) from two BrS patients (BrS-CMs) carrying a heterozygous SCN5A mutation p.S1812X. Compared to CMs derived from healthy controls (Ctrl-CMs), BrS-CMs displayed a 50% reduction of I Na density, a 69.5% reduction of NaV1.5 expression, and the impaired localization of NaV1.5 and connexin 43 (Cx43) at the cell surface. BrS-CMs exhibited reduced action potential (AP) upstroke velocity and conduction slowing. The I to in BrS-CMs was significantly augmented, and the I CaL window current probability was increased. Our data indicate that the electrophysiological mechanisms underlying arrhythmia in BrS-CMs may involve both depolarization and repolarization disorders. Cilostazol and milrinone showed dramatic inhibitions of I to in BrS-CMs and alleviated the arrhythmic activity, suggesting their therapeutic potential for BrS patients.
Keywords: Brugada syndrome; SCN5A mutation; depolarization; disease modeling; induced pluripotent stem cells; repolarization.
Copyright © 2020 Li, Stauske, Luo, Wagner, Vollrath, Mehnert, Schubert, Cyganek, Chen, Hasheminasab, Wulf, El-Armouche, Maier, Hasenfuss and Guan.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

