Intracellular Transport in Cancer Metabolic Reprogramming
- PMID: 33195279
- PMCID: PMC7661548
- DOI: 10.3389/fcell.2020.597608
Intracellular Transport in Cancer Metabolic Reprogramming
Abstract
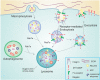
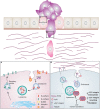
Tumor progression is a complex process consisting of several steps characterized by alterations in cellular behavior and morphology. These steps include uncontrolled cell division and proliferation, invasiveness and metastatic ability. Throughout these phases, cancer cells encounter a changing environment and a variety of metabolic stress. To meet their needs for energy while they proliferate and survive in their new environment, tumor cells need to continuously fine-tune their metabolism. The connection between intracellular transport and metabolic reprogramming during cancer progression is emerging as a central process of cellular adaptation to these changes. The trafficking of proteolytic enzymes, surface receptors, but also the regulation of downstream pathways, are all central to cancer progression. In this review, we summarize different hallmarks of cancer with a special focus on the role of intracellular trafficking in cell proliferation, epithelial to mesenchymal transition as well as invasion. We will further emphasize how intracellular trafficking contributes to the regulation of energy consumption and metabolism during these steps of cancer progression.
Keywords: cancer cell metabolism; cell proliferation; epithelial to mesenchymal transition; invasion; membrane trafficking.
Copyright © 2020 Sneeggen, Guadagno and Progida.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

