IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections
- PMID: 33195334
- PMCID: PMC7655919
- DOI: 10.3389/fmed.2020.583897
IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections
Abstract
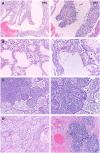
Background: Anti-inflammatory therapies such as IL-6 inhibition have been proposed for COVID-19 in a vacuum of evidence-based treatment. However, abrogating the inflammatory response in infectious diseases may impair a desired host response and pre-dispose to secondary infections. Methods: We retrospectively reviewed the medical record of critically ill COVID-19 patients during an 8-week span and compared the prevalence of secondary infection and outcomes in patients who did and did not receive tocilizumab. Additionally, we included representative histopathologic post-mortem findings from several COVID-19 cases that underwent autopsy at our institution. Results: One hundred eleven patients were identified, of which 54 had received tocilizumab while 57 had not. Receiving tocilizumab was associated with a higher risk of secondary bacterial (48.1 vs. 28.1%; p = 0.029 and fungal (5.6 vs. 0%; p = 0.112) infections. Consistent with higher number of infections, patients who received tocilizumab had higher mortality (35.2 vs. 19.3%; p = 0.020). Seven cases underwent autopsy. In three cases who received tocilizumab, there was evidence of pneumonia on pathology. Of the four cases that had not been given tocilizumab, two showed evidence of aspiration pneumonia and two exhibited diffuse alveolar damage. Conclusions: Experimental therapies are currently being applied to COVID-19 outside of clinical trials. Anti-inflammatory therapies such as anti-IL-6 therapy have the potential to impair viral clearance, pre-dispose to secondary infection, and cause harm. We seek to raise physician awareness of these issues and highlight the need to better understand the immune response in COVID-19.
Keywords: COVID-19; SARS-CoV-2; cytokine release syndrome; immunosuppression; tocilizumab.
Copyright © 2020 Kimmig, Wu, Gold, Pettit, Pitrak, Mueller, Husain, Mutlu and Mutlu.
Figures

Update of
-
IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections.medRxiv [Preprint]. 2020 Sep 12:2020.05.15.20103531. doi: 10.1101/2020.05.15.20103531. medRxiv. 2020. Update in: Front Med (Lausanne). 2020 Oct 28;7:583897. doi: 10.3389/fmed.2020.583897. PMID: 32935118 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

