Short Chain Fatty Acids Enhance Expression and Activity of the Umami Taste Receptor in Enteroendocrine Cells via a Gαi/o Pathway
- PMID: 33195366
- PMCID: PMC7658341
- DOI: 10.3389/fnut.2020.568991
Short Chain Fatty Acids Enhance Expression and Activity of the Umami Taste Receptor in Enteroendocrine Cells via a Gαi/o Pathway
Abstract
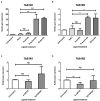
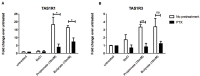
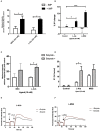
The short chain fatty acids (SCFAs) acetate, butyrate and propionate, are produced by fermentation of non-digestible carbohydrates by the gut microbiota and regulate appetite, adiposity, metabolism, glycemic control, and immunity. SCFAs act at two distinct G protein coupled receptors (GPCRs), FFAR2 and FFAR3 and are expressed in intestinal enteroendocrine cells (EECs), where they mediate anorectic gut hormone release. EECs also express other GPCRs that act as nutrient sensors, thus SCFAs may elicit some of their health-promoting effects by altering GPCR expression in EECs and enhance gut sensitivity to dietary molecules. Here, we identify that exposure of the murine EEC STC-1 cell line or intestinal organoids to physiological concentrations of SCFAs enhances mRNA levels of the umami taste receptors TASR1 and TASR3, without altering levels of the SCFA GPCRs, FFAR2 and FFAR3. Treatment of EECs with propionate or butyrate, but not acetate, increased levels of umami receptor transcripts, while propionate also reduced CCK expression. This was reversed by inhibiting Gαi/o signaling with pertussis toxin, suggesting that SCFAs act through FFAR2/3 to alter gene expression. Surprisingly, neither a FFAR3 nor a FFAR2 selective ligand could increase TASR1/TASR3 mRNA levels. We assessed the functional impact of increased TASR1/TASR3 expression using unique pharmacological properties of the umami taste receptor; namely, the potentiation of signaling by inosine monophosphate. Activation of umami taste receptor induced inositol-1-phosphate and calcium signaling, and butyrate pretreatment significantly enhanced such signaling. Our study reveals that SCFAs may contribute to EEC adaptation and alter EEC sensitivity to bioactive nutrients.
Keywords: GPCR (G protein coupled receptor); enteroendocrine cell; heterotrimeric G protein; short chain fatty acid (SCFA); umami taste receptor.
Copyright © 2020 Shackley, Ma, Tate, Brown, Frost and Hanyaloglu.
Figures

References
-
- Hijova E, Chmelarova A. Short chain fatty acids and colonic health. Bratisl Lek Listy. (2007) 108:354. Available online at: http://bmj.fmed.uniba.sk/2007/10808-06.pdf - PubMed
-
- Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SEK, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. (2015) 64:1744–54. 10.1136/gutjnl-2014-307913 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

