Enzyme Kinetics by Isothermal Titration Calorimetry: Allostery, Inhibition, and Dynamics
- PMID: 33195429
- PMCID: PMC7604385
- DOI: 10.3389/fmolb.2020.583826
Enzyme Kinetics by Isothermal Titration Calorimetry: Allostery, Inhibition, and Dynamics
Abstract
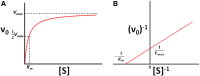
Isothermal titration calorimetry (ITC) involves accurately measuring the heat that is released or absorbed in real time when one solution is titrated into another. This technique is usually used to measure the thermodynamics of binding reactions. However, there is mounting interest in using it to measure reaction kinetics, particularly enzymatic catalysis. This application of ITC has been steadily growing for the past two decades, and the method is proving to be sensitive, generally applicable, and capable of providing information on enzyme activity that is difficult to obtain using traditional biochemical assays. This review aims to give a broad overview of the use of ITC to measure enzyme kinetics. It describes several different classes of ITC experiment, their strengths and weaknesses, and recent methodological advancements. A summary of applications in the literature is given and several examples where ITC has been used to investigate challenging aspects of enzyme behavior are presented in more detail. These include examples of allostery, where small-molecule binding outside the active site modulates activity. We describe the use of ITC to measure the strength, mode (i.e., competitive, uncompetitive, or mixed), and association and dissociation kinetics of enzyme inhibitors. Further, we provide examples of ITC applied to complex, heterogeneous mixtures, such as insoluble substrates and live cells. These studies exemplify the wide range of problems where ITC can provide answers, and illustrate the versatility of the technique and potential for future development and applications.
Keywords: ITC; activation; allostery; enzyme catalysis; inhibition; kinetics.
Copyright © 2020 Wang, Wang, Moitessier and Mittermaier.
Figures




Similar articles
-
Isothermal Titration Calorimetry to Characterize Enzymatic Reactions.Methods Enzymol. 2016;567:215-36. doi: 10.1016/bs.mie.2015.07.022. Epub 2015 Sep 1. Methods Enzymol. 2016. PMID: 26794356
-
Thermodynamics of protein-ligand interactions: history, presence, and future aspects.J Recept Signal Transduct Res. 2004 Feb;24(1-2):1-52. doi: 10.1081/rrs-120037896. J Recept Signal Transduct Res. 2004. PMID: 15344878 Review.
-
Complete Kinetic Characterization of Enzyme Inhibition in a Single Isothermal Titration Calorimetric Experiment.Anal Chem. 2018 Jul 17;90(14):8430-8435. doi: 10.1021/acs.analchem.8b00993. Epub 2018 Jul 6. Anal Chem. 2018. PMID: 29926719
-
Characterization of Enzymatic Reactions Using ITC.Methods Mol Biol. 2019;1964:251-266. doi: 10.1007/978-1-4939-9179-2_18. Methods Mol Biol. 2019. PMID: 30929248
-
Applications of isothermal titration calorimetry in pure and applied research--survey of the literature from 2010.J Mol Recognit. 2012 Jan;25(1):32-52. doi: 10.1002/jmr.1167. J Mol Recognit. 2012. PMID: 22213449 Review.
Cited by
-
Novel sensor-integrated proteome on chip (SPOC) platform with thousands of folded proteins on a 1.5 sq-cm biosensor chip to enable high-throughput real-time label-free screening for kinetic analysis.bioRxiv [Preprint]. 2024 Jan 24:2024.01.23.575909. doi: 10.1101/2024.01.23.575909. bioRxiv. 2024. Update in: Commun Biol. 2025 Mar 21;8(1):468. doi: 10.1038/s42003-025-07844-z. PMID: 38328216 Free PMC article. Updated. Preprint.
-
A FRET-Based Assay and Computational Tools to Quantify Enzymatic Rates and Explore the Mechanisms of RNA Deadenylases in Heterogeneous Environments.Methods Mol Biol. 2024;2723:69-91. doi: 10.1007/978-1-0716-3481-3_5. Methods Mol Biol. 2024. PMID: 37824065
-
Applying Isothermal Titration Calorimetry and Saturation Transfer Difference-NMR to Study the Mode of Interaction of Flavan-3-ols with α-Amylase to Understand Their Impact on Starch Hydrolysis.J Agric Food Chem. 2025 Apr 16;73(15):9047-9061. doi: 10.1021/acs.jafc.4c13178. Epub 2025 Apr 4. J Agric Food Chem. 2025. PMID: 40184499 Free PMC article.
-
Kinetic Analysis of Prostate-Specific Antigen Interaction with Monoclonal Antibodies for Development of a Magnetic Immunoassay Based on Nontransparent Fiber Structures.Molecules. 2022 Nov 21;27(22):8077. doi: 10.3390/molecules27228077. Molecules. 2022. PMID: 36432177 Free PMC article.
-
Advances in Computational Methods to Discover New NS2B-NS3 Inhibitors Useful Against Dengue and Zika Viruses.Curr Top Med Chem. 2022;22(29):2435-2462. doi: 10.2174/1568026623666221122121330. Curr Top Med Chem. 2022. PMID: 36415099 Review.
References
-
- Abdel-Hamid A. M., Solbiati J. O., Cann I. K. O. (2013). “Insights into lignin degradation and its potential industrial applications,” in Advances in Applied Microbiology, Vol. 82 eds Sariaslani S., Gadd G. M. (Cambridge, MA: Academic Press; ), 1–28. 10.1016/b978-0-12-407679-2.00001-6 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

