Influenza A Virus in Swine: Epidemiology, Challenges and Vaccination Strategies
- PMID: 33195504
- PMCID: PMC7536279
- DOI: 10.3389/fvets.2020.00647
Influenza A Virus in Swine: Epidemiology, Challenges and Vaccination Strategies
Abstract
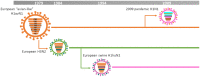
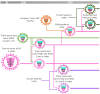
Influenza A viruses cause acute respiratory infections in swine that result in significant economic losses for global pig production. Currently, three different subtypes of influenza A viruses of swine (IAV-S) co-circulate worldwide: H1N1, H3N2, and H1N2. However, the origin, genetic background and antigenic properties of those IAV-S vary considerably from region to region. Pigs could also have a role in the adaptation of avian influenza A viruses to humans and other mammalian hosts, either as intermediate hosts in which avian influenza viruses may adapt to humans, or as a "mixing vessel" in which influenza viruses from various origins may reassort, generating novel progeny viruses capable of replicating and spreading among humans. These potential roles highlight the importance of controlling influenza A viruses in pigs. Vaccination is currently the main tool to control IAV-S. Vaccines containing whole inactivated virus (WIV) with adjuvant have been traditionally used to generate highly specific antibodies against hemagglutinin (HA), the main antigenic protein. WIV vaccines are safe and protect against antigenically identical or very similar strains in the absence of maternally derived antibodies (MDAs). Yet, their efficacy is reduced against heterologous strains, or in presence of MDAs. Moreover, vaccine-associated enhanced respiratory disease (VAERD) has been described in pigs vaccinated with WIV vaccines and challenged with heterologous strains in the US. This, together with the increasingly complex epidemiology of SIVs, illustrates the need to explore new vaccination technologies and strategies. Currently, there are two different non-inactivated vaccines commercialized for swine in the US: an RNA vector vaccine expressing the HA of a H3N2 cluster IV, and a bivalent modified live vaccine (MLV) containing H1N2 γ-clade and H3N2 cluster IV. In addition, recombinant-protein vaccines, DNA vector vaccines and alternative attenuation technologies are being explored, but none of these new technologies has yet reached the market. The aim of this article is to provide a thorough review of the current epidemiological scenario of IAV-S, the challenges faced in the control of IAV-S infection and the tools being explored to overcome those challenges.
Keywords: epidemology; influenza A; role of pig; swine; vaccines.
Copyright © 2020 Mancera Gracia, Pearce, Masic and Balasch.
Figures
Similar articles
-
Comparison of Adjuvanted-Whole Inactivated Virus and Live-Attenuated Virus Vaccines against Challenge with Contemporary, Antigenically Distinct H3N2 Influenza A Viruses.J Virol. 2018 Oct 29;92(22):e01323-18. doi: 10.1128/JVI.01323-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30185589 Free PMC article.
-
Vaccine-Associated Enhanced Respiratory Disease following Influenza Virus Infection in Ferrets Recapitulates the Model in Pigs.J Virol. 2022 Mar 9;96(5):e0172521. doi: 10.1128/JVI.01725-21. Epub 2022 Jan 5. J Virol. 2022. PMID: 34985999 Free PMC article.
-
Heterologous prime-boost H1N1 vaccination exacerbates disease following challenge with a mismatched H1N2 influenza virus in the swine model.Front Immunol. 2023 Oct 20;14:1253626. doi: 10.3389/fimmu.2023.1253626. eCollection 2023. Front Immunol. 2023. PMID: 37928521 Free PMC article.
-
[Swine influenza virus: evolution mechanism and epidemic characterization--a review].Wei Sheng Wu Xue Bao. 2009 Sep;49(9):1138-45. Wei Sheng Wu Xue Bao. 2009. PMID: 20030049 Review. Chinese.
-
Pathogenesis and vaccination of influenza A virus in swine.Curr Top Microbiol Immunol. 2014;385:307-26. doi: 10.1007/82_2014_391. Curr Top Microbiol Immunol. 2014. PMID: 25033752 Review.
Cited by
-
Protein sequence features of H1N1 swine influenza A viruses detected on commercial swine farms in Serbia.J Vet Res. 2023 Jun 16;67(2):147-154. doi: 10.2478/jvetres-2023-0034. eCollection 2023 Jun. J Vet Res. 2023. PMID: 38143831 Free PMC article.
-
Swine influenza A virus: challenges and novel vaccine strategies.Front Cell Infect Microbiol. 2024 Apr 3;14:1336013. doi: 10.3389/fcimb.2024.1336013. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38633745 Free PMC article. Review.
-
Animal Models Utilized for the Development of Influenza Virus Vaccines.Vaccines (Basel). 2021 Jul 14;9(7):787. doi: 10.3390/vaccines9070787. Vaccines (Basel). 2021. PMID: 34358203 Free PMC article. Review.
-
Estimating the risk of zoonotic transmission of swine influenza A variant during agricultural fairs in the United States: a mathematical modeling.Front Vet Sci. 2025 Apr 1;12:1523981. doi: 10.3389/fvets.2025.1523981. eCollection 2025. Front Vet Sci. 2025. PMID: 40235572 Free PMC article.
-
Ecological and evolutionary dynamics of multi-strain RNA viruses.Nat Ecol Evol. 2022 Oct;6(10):1414-1422. doi: 10.1038/s41559-022-01860-6. Epub 2022 Sep 22. Nat Ecol Evol. 2022. PMID: 36138206 Review.
References
-
- Van Reeth K, Vincent AL. Influenza viruses. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J. editors. Diseases of Swine. Ames, IA: John Willey and Sons Inc; (2019). p. 480–504.
-
- Qiu Y. Cross-protection studies with swine influenza viruses in pigs and public health aspects (PhD thesis). University of Ghent, Ghent, Belgium (2015).
Publication types
LinkOut - more resources
Full Text Sources