Diagnostic Imaging in Intervertebral Disc Disease
- PMID: 33195623
- PMCID: PMC7642913
- DOI: 10.3389/fvets.2020.588338
Diagnostic Imaging in Intervertebral Disc Disease
Abstract
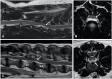
Imaging is integral in the diagnosis of canine intervertebral disc disease (IVDD) and in differentiating subtypes of intervertebral disc herniation (IVDH). These include intervertebral disc extrusion (IVDE), intervertebral disc protrusion (IVDP) and more recently recognized forms such as acute non-compressive nucleus pulposus extrusion (ANNPE), hydrated nucleus pulposus extrusion (HNPE), and intradural/intramedullary intervertebral disc extrusion (IIVDE). Many imaging techniques have been described in dogs with roles for survey radiographs, myelography, computed tomography (CT), and magnetic resonance imaging (MRI). Given how common IVDH is in dogs, a thorough understanding of the indications and limitations for each imaging modality to aid in diagnosis, treatment planning and prognosis is essential to successful case management. While radiographs can provide useful information, especially for identifying intervertebral disc degeneration or calcification, there are notable limitations. Myelography addresses some of the constraints of survey radiographs but has largely been supplanted by cross-sectional imaging. Computed tomography with or without myelography and MRI is currently utilized most widely and have become the focus of most contemporary studies on this subject. Novel advanced imaging applications are being explored in dogs but are not yet routinely performed in clinical patients. The following review will provide a comprehensive overview on common imaging modalities reported to aid in the diagnosis of IVDH including IVDE, IVDP, ANNPE, HNPE, and IIVDE. The review focuses primarily on canine IVDH due to its frequency and vast literature as opposed to feline IVDH.
Keywords: computed tomography; extrusion; herniation; intervertebral disc; magnetic resonance imaging; protrusion.
Copyright © 2020 da Costa, De Decker, Lewis, Volk and the Canine Spinal Cord Injury Consortium (CANSORT-SCI).
Figures
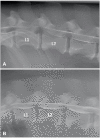


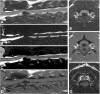




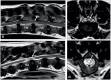

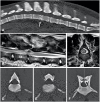



References
-
- Priester WA. Canine intervertebral disc disease – occurrence by age, breed, and sex among 8,117 cases. Theriogenology. (1976) 6:293–303. 10.1016/0093-691X(76)90021-2 - DOI

