Predictive biomarkers for sacituzumab govitecan efficacy in Trop-2-expressing triple-negative breast cancer
- PMID: 33196706
- PMCID: PMC7597411
- DOI: 10.18632/oncotarget.27766
Predictive biomarkers for sacituzumab govitecan efficacy in Trop-2-expressing triple-negative breast cancer
Abstract
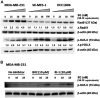
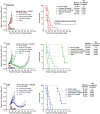
Sacituzumab govitecan (SG) is an antibody-drug conjugate composed of a humanized anti-Trop-2 IgG antibody conjugated via a hydrolysable linker to SN-38, the topoisomerase I-inhibitory active component of irinotecan. We investigated whether Trop-2-expression and homologous recombination repair (HRR) of SN-38-mediated double-strand DNA (dsDNA) breaks play a role in the sensitivity of triple-negative breast cancer (TNBC) to SG. Activation of HRR pathways, as evidenced by Rad51 expression, was assessed in SG-sensitive cell lines with low and moderate Trop-2-expression (SK-MES-1 squamous cell lung carcinoma and HCC1806 TNBC, respectively), compared to a low Trop-2-expressing, less SG-sensitive TNBC cell line (MDA-MB-231). Further, two Trop-2-transfectants of MDA-MB-231, C13 and C39 (4- and 25-fold higher Trop-2, respectively), were treated in mice with SG to determine whether increasing Trop-2 expression improves SG efficacy. SG mediated >2-fold increase in Rad51 in MDA-MB-231 but had no effect in SK-MES-1 or HCC1806, resulting in lower levels of dsDNA breaks in MDA-MB-231. SG and saline produced similar effects in parental MDA-MB-231 tumor-bearing mice (median survival time (MST) = 21d and 19.5d, respectively). However, in mice bearing higher Trop-2-expressing C13 and C39 tumors after Trop-2 transfection, SG provided a significant survival benefit, even compared to irinotecan (MST = 97d vs. 35d for C13, and 81d vs. 28d for C39, respectively; P < 0.0007). These results suggest that SG could provide better clinical benefit than irinotecan in patients with HRR-proficient tumors expressing high levels of Trop-2, as well as to patients with HRR-deficient tumors expressing low/moderate levels of Trop-2.
Keywords: RAD51; Trop-2; biomarker; sacituzumab govitecan; triple-negative breast cancer.
Copyright: © 2020 Cardillo et al.
Conflict of interest statement
CONFLICTS OF INTEREST All authors are employed or were employed by Immunomedics, Inc., and hold stock or stock options in Immunomedics, Inc. David M. Goldenberg holds patents with potential royalties. David M. Goldenberg is an inventor of numerous relevant patents on sacituzumab govitecan.
Figures


References
-
- Ramaker RC, Lasseigne BN, Hardigan AA, Palacio L, Gunther DS, Myers RM, Cooper SJ. RNA sequencing-based cell proliferation analysis across 19 cancers identifies a subset of proliferation-informative cancers with a common survival signature. Oncotarget. 2017; 8:38668–38681. 10.18632/oncotarget.16961. - DOI - PMC - PubMed
-
- Schink JC, Trosman JR, Weldon CB, Siziopikou KP, Tsongalis GJ, Rademaker AW, Patel JD, Benson AB 3rd, Perez EA, Gradishar WJ. Biomarker testing for breast, lung, and gastroesophageal cancers at NCI designated cancer centers. J Natl Cancer Inst. 2014; 106:dju256. 10.1093/jnci/dju256. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

