Mitochondria-ER Tethering in Neurodegenerative Diseases
- PMID: 33196974
- PMCID: PMC11441217
- DOI: 10.1007/s10571-020-01008-9
Mitochondria-ER Tethering in Neurodegenerative Diseases
Abstract
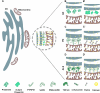
Organelles juxtaposition has been detected for decades, although only recently gained importance due to a pivotal role in the regulation of cellular processes dependent on membrane contact sites. Endoplasmic reticulum (ER) and mitochondria interaction is a prime example of organelles contact sites. Mitochondria-associated membranes (MAM) are proposed to harbor ER-mitochondria tether complexes, mainly when these organelles are less than 30 nm apart. Dysfunctions of proteins located at the MAM are associated with neurodegenerative diseases such as Parkinson's, Alzheimer's and amyotrophic lateral sclerosis, as well as neurodevelopmental disorders; hence any malfunction in MAM can potentially trigger cell death. This review will focus on the role of ER-mitochondria contact sites, regarding calcium homeostasis, lipid metabolism, autophagy, morphology and dynamics of mitochondria, mainly in the context of neurodegenerative diseases. Approaches that have been employed so far to study organelles contact sites, as well as methods that were not used in neurosciences yet, but are promising and accurate ways to unveil the functions of MAM during neurodegeneration, is also discussed in the present review.
Keywords: Autophagy; Calcium; Contact sites methodologies; Lipid metabolism; Mitochondria-associated membranes (MAM); Neurodegeneration.
© 2020. Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- AlSaif A, AlMohanna F, Bohlega S (2011) A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol 70:913–919. 10.1002/ana.22534 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

