Does additional monitoring status increase the reporting of adverse drug reactions? An interrupted time series analysis of EudraVigilance data
- PMID: 33197106
- PMCID: PMC7898803
- DOI: 10.1002/pds.5174
Does additional monitoring status increase the reporting of adverse drug reactions? An interrupted time series analysis of EudraVigilance data
Abstract
Purpose: To evaluate the impact of including a medicine in the list of medicinal products subject to additional monitoring (AM) on the reporting of adverse drug reactions (ADRs) in the european economic area (EEA).
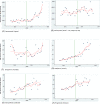
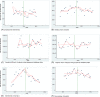
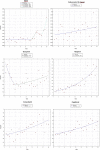
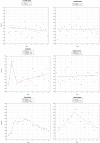
Methods: Interrupted time series using the monthly number of EEA ADR reports in EudraVigilance during 12 months before and after the addition to AM list. The main outcome was the change (%) in reporting of ADRs with step change as the a priori impact model. Further time series analysis was performed using Joinpoint Regression.
Results: The analysis included 11 active substances. No significant immediate (step change) increase of reporting was identified for any product at time of addition to AM list. We identified a significant gradual increase of ADR reporting after addition to AM list (slope change) for two out of five new products-boceprevir (10% per month, 95% confidence interval (CI) 3%-18%) and denosumab-Xgeva (13% per month, 95% CI 4%-22%). No change was identified for Prolia, another denosumab-containing product not subject to AM. No significant increase was identified for any product included in the AM list due to the requirement to conduct a PASS. Conversely, a gradual decrease in reporting was identified for natalizumab (-5% per month; 95% CI -10% to -1%), rivaroxaban (-5%; -8 to -3%), and varenicline (-16%; -21 to -10%). The results were corroborated by the Joinpoint analyses, which yielded similar results.
Conclusions: We identified limited evidence that reporting of ADRs increased modestly and gradually for some new products and not for products with PASS requirement.
Keywords: additional monitoring; adverse drug reactions; impact; pharmacovigilance.
© 2020 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

References
-
- Regulation (EC) No 726/2004 of the European Parliament and of the council of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency, Official Journal L – 136, 30/04/2004, p. 1–33. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:02004R0726-201.... Accessed April 30, 2019.
-
- European Medicines Agency . List of medicines subject to additional monitoring, EMA/245297/2013 Rev.60. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmac.... Accessed January 17, 2020.
-
- European Medicines Agency . Guideline on good pharmacovigilance practices Module X – additional monitoring. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed April 30, 2019.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

