Critical Steps in Data Management During a Crisis
- PMID: 33197114
- PMCID: PMC7753671
- DOI: 10.1002/cyto.a.24265
Critical Steps in Data Management During a Crisis
Abstract
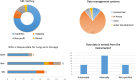
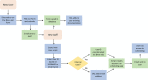
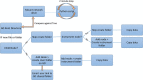
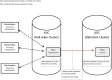
Data management is essential in a flow cytometry (FCM) shared resource laboratory (SRL) for the integrity of collected data and its long-term preservation, as described in the Cytometry publication from 2016, ISAC Flow Cytometry Shared Resource Laboratory (SRL) Best Practices (Barsky et al.: Cytometry Part A 89A(2016): 1017-1030). The SARS-CoV-2 pandemic introduced an array of challenges in the operation of SRLs. The subsequent laboratory shutdowns and access restrictions brought to the forefront well-established practices that withstood the impact of a sudden change in operations and illuminated areas that need improvement. The most significant challenges from a data management perspective were data access for remote analysis and workstation management. Notably, lessons learned from this challenge emphasize the importance of safeguarding collected data from loss in various emergencies such as fire or natural disasters where the physical hardware storing data could be directly affected. Here, we describe two data management systems that have been successful during the current emergency created by the pandemic, specifically remote access and automated data transfer. We will discuss other situations that could arise and lead to data loss or challenges in interpreting data. © 2020 International Society for Advancement of Cytometry.
Keywords: SRL; best practices; data management; flow cytometry shared resource lab; laboratory preparedness.
© 2020 International Society for Advancement of Cytometry.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures
References
-
- National Research Council (US) Committee on Prudent Practices in the Laboratory . Prudent Practices in the Laboratory: Handling and Management of Chemical Hazards: Updated Version. Washington (DC): National Academies Press (US), 2011. Available at: https://www.ncbi.nlm.nih.gov/books/NBK55874/. - PubMed
-
- Krane N, Kevin MD, Kahn MJ, Markert RJ, Whelton PK, Traber PG, Taylor IL. Surviving Hurricane Katrina: Reconstructing the Educational Enterprise of Tulane University School of Medicine. Acad Med 2007;82(8):757–762. - PubMed
-
- ISAC Shared Resource Lab Services Committee. SRL COVID‐19 survey results. https://cdn.ymaws.com/isac-net.org/resource/resmgr/docs/srl_newsletter/s...
-
- United States Department of Health & Human Services . Office for Civil Rights Privacy Brief: Summary of the HIPAA Privacy Rule, HIPAA Compliance Assistance. Available at: http://www.hhs.gov/sites/default/files/privacysummary.pdf
-
- Spidlen J, Brinkman RR. Use flow repository to share your clinical data upon study publication. Cytom Part B 2018;94B:196–198. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

