NHC-stabilized Parent Arsanylalanes and -gallanes
- PMID: 33197127
- PMCID: PMC7898810
- DOI: 10.1002/anie.202013849
NHC-stabilized Parent Arsanylalanes and -gallanes
Abstract
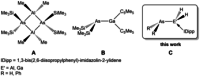
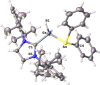
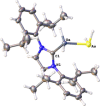
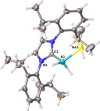
The synthesis and characterization of the unprecedented compounds IDipp⋅E'H2 AsH2 (E'=Al, Ga; IDipp=1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene) are reported, the first monomeric, parent representatives of an arsanylalane and arsanylgallane, respectively, stabilized only by a LB (LB=Lewis Base). They are prepared by a salt metathesis reaction of KAsH2 with IDipp⋅E'H2 Cl (E'=Al, Ga). The H2 -elimination pathway through the reaction of AsH3 with IDipp⋅E'H3 (E'=Al, Ga) was found to be a possible synthetic route with some disadvantages compared to the salt metathesis reaction. The corresponding organo-substituted compounds IDipp⋅GaH2 AsPh2 (1) and IDipp⋅AlH2 AsPh2 (2) were obtained by the reaction of KAsPh2 with IDipp⋅E'H2 Cl (E'=Al, Ga). The novel branched parent compounds IDipp⋅E'H(EH2 )2 (E'=Al, Ga; E=P, As) were synthesized by salt metathesis reactions starting from IDipp⋅E'HCl2 (E'=Al, Ga). Supporting DFT computations give insight into the different synthetic pathways and the stability of the products.
Keywords: Lewis bases; alanes; arsenic; gallanes; group 13/15 compounds.
© 2020 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- None
-
- Masuda J. D., Hoshkin A. J., Graham T. W., Beddic C., Fermin M. C., Etkin N., Stephan D. W., Chem. Eur. J. 2006, 12, 8696–8707; - PubMed
-
- Fischer R. A., Weiß J., Angew. Chem. Int. Ed. 1999, 38, 2830–2850; - PubMed
- Angew. Chem. 1999, 111, 3002–3022;
-
- Wells R. L., Gladfelter W. L., J. Cluster Sci. 1997, 8, 217–238;
-
- Jones A. C., O'Brien P., in CVD of Compound Semiconductors: Precursor Synthesis Development and Applications, VCH, Weinheim, 1996.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

