Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function
- PMID: 33197224
- PMCID: PMC8017904
- DOI: 10.1146/annurev-physiol-031620-095920
Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function
Abstract
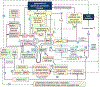
SGLT2 inhibitors are antihyperglycemic drugs that protect kidneys and the heart of patients with or without type 2 diabetes and preserved or reduced kidney function from failing. The involved protective mechanisms include blood glucose-dependent and -independent mechanisms: SGLT2 inhibitors prevent both hyper- and hypoglycemia, with expectedly little net effect on HbA1C. Metabolic adaptations to induced urinary glucose loss include reduced fat mass and more ketone bodies as additional fuel. SGLT2 inhibitors lower glomerular capillary hypertension and hyperfiltration, thereby reducing the physical stress on the filtration barrier, albuminuria, and the oxygen demand for tubular reabsorption. This improves cortical oxygenation, which, together with lesser tubular gluco-toxicity, may preserve tubular function and glomerular filtration rate in the long term. SGLT2 inhibitors may mimic systemic hypoxia and stimulate erythropoiesis, which improves organ oxygen delivery. SGLT2 inhibitors are proximal tubule and osmotic diuretics that reduce volume retention and blood pressure and preserve heart function, potentially in part by overcoming the resistance to diuretics and atrial-natriuretic-peptide and inhibiting Na-H exchangers and sympathetic tone.
Keywords: HFpEF; HFrEF; SGLT2 inhibitor; chronic kidney disease; diabetic nephropathy; heart failure.
Figures


References
-
- Food US and Admin Drug. 2008. Guidance for industry on diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes; availability. Fed. Regist 73. https://www.govinfo.gov/content/pkg/FR-2008-12-19/pdf/E8-30086.pdf
-
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, et al. 2019. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med 380:347–57 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

