A multimodal and integrated approach to interrogate human kidney biopsies with rigor and reproducibility: guidelines from the Kidney Precision Medicine Project
- PMID: 33197228
- PMCID: PMC7847045
- DOI: 10.1152/physiolgenomics.00104.2020
A multimodal and integrated approach to interrogate human kidney biopsies with rigor and reproducibility: guidelines from the Kidney Precision Medicine Project
Abstract
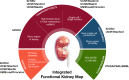
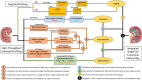
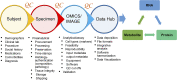
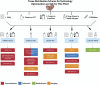
Comprehensive and spatially mapped molecular atlases of organs at a cellular level are a critical resource to gain insights into pathogenic mechanisms and personalized therapies for diseases. The Kidney Precision Medicine Project (KPMP) is an endeavor to generate three-dimensional (3-D) molecular atlases of healthy and diseased kidney biopsies by using multiple state-of-the-art omics and imaging technologies across several institutions. Obtaining rigorous and reproducible results from disparate methods and at different sites to interrogate biomolecules at a single-cell level or in 3-D space is a significant challenge that can be a futile exercise if not well controlled. We describe a "follow the tissue" pipeline for generating a reliable and authentic single-cell/region 3-D molecular atlas of human adult kidney. Our approach emphasizes quality assurance, quality control, validation, and harmonization across different omics and imaging technologies from sample procurement, processing, storage, shipping to data generation, analysis, and sharing. We established benchmarks for quality control, rigor, reproducibility, and feasibility across multiple technologies through a pilot experiment using common source tissue that was processed and analyzed at different institutions and different technologies. A peer review system was established to critically review quality control measures and the reproducibility of data generated by each technology before their being approved to interrogate clinical biopsy specimens. The process established economizes the use of valuable biopsy tissue for multiomics and imaging analysis with stringent quality control to ensure rigor and reproducibility of results and serves as a model for precision medicine projects across laboratories, institutions and consortia.
Keywords: imaging; kidney disease; metabolomics; proteomics; transcriptomics.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

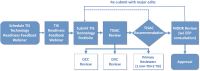
References
-
- Chen L, Lee JW, Chou C-L, Nair AV, Battistone MA, Păunescu TG, Merkulova M, Breton S, Verlander JW, Wall SM, Brown D, Burg MB, Knepper MA. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci USA 114: E9989–E9998, 2017. doi:10.1073/pnas.1710964114. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- UH3 DK114907/DK/NIDDK NIH HHS/United States
- K08 DK107864/DK/NIDDK NIH HHS/United States
- U2C DK114886/DK/NIDDK NIH HHS/United States
- UH3 DK114920/DK/NIDDK NIH HHS/United States
- UG3 DK114920/DK/NIDDK NIH HHS/United States
- U01 DK114933/DK/NIDDK NIH HHS/United States
- U54 HL145608/HL/NHLBI NIH HHS/United States
- P30 DK079312/DK/NIDDK NIH HHS/United States
- R01 GM071966/GM/NIGMS NIH HHS/United States
- U01 DK114907/DK/NIDDK NIH HHS/United States
- U01 DK114920/DK/NIDDK NIH HHS/United States
- UH3 DK114933/DK/NIDDK NIH HHS/United States
- U24 DK114886/DK/NIDDK NIH HHS/United States
- UG3 DK114933/DK/NIDDK NIH HHS/United States
- UH3 DK114923/DK/NIDDK NIH HHS/United States
- UH3 DK114937/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

