Complications related to elective generator replacement of the subcutaneous implantable defibrillator
- PMID: 33197266
- PMCID: PMC7947576
- DOI: 10.1093/europace/euaa263
Complications related to elective generator replacement of the subcutaneous implantable defibrillator
Abstract
Aims: To guarantee uninterrupted function of the subcutaneous implantable cardioverter-defibrillator (S-ICD), the pulse generator needs to be surgically replaced before the battery is depleted. The risks related to this replacement substantially impact long-term outcome for S-ICD recipients, as the majority will undergo one or several of these procedures in their lifetime. We aim to describe the procedural characteristics of the replacement procedure and to provide an insight in the complications associated with these replacements.
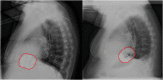
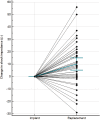
Methods and results: In this retrospective analysis, data from replacement procedures and follow-up visits were collected from all patients who underwent elective S-ICD generator replacement in our tertiary centre from June 2014 until November 2019. Original device position was assessed using the PRAETORIAN score. Complications were defined as those requiring surgical intervention, systemic antibiotic treatment, or device extraction. Seventy-two patients were included, with a median follow-up of 1.9 years (IQR 0.6-3.3 years) after replacement. Battery depletion occurred after 5.9 ± 0.7 years. The pulse generator was repositioned in patients with a PRAETORIAN score ≥90 to minimize the defibrillation threshold. Although there was an increase in impedance compared to the implant procedure, first shock conversion rate during defibrillation testing was 91.4% with a success rate of 100% after multiple attempts. Two patients developed a complication after, respectively, 9 and 21 months, resulting in a complication rate of 1.4% per year.
Conclusion: With a median follow-up of 1.9 years, this study shows a low complication rate after S-ICD replacement, with a first shock conversion rate of 91.4%.
Keywords: Battery longevity; Complication rate; PRAETORIAN score; Pulse generator replacement; Shock impedance; Subcutaneous implantable cardioverter-defibrillator.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
Comment in
-
High shock impedance during subcutaneous implantable defibrillator generator replacements: Authors' reply.Europace. 2022 Feb 2;24(2):350-351. doi: 10.1093/europace/euab205. Europace. 2022. PMID: 34626183 Free PMC article. No abstract available.
-
High shock impedance during subcutaneous implantable defibrillator generator replacements.Europace. 2022 Feb 2;24(2):349-350. doi: 10.1093/europace/euab203. Europace. 2022. PMID: 34626189 No abstract available.
References
-
- Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L. et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med 2010;363:36–44. - PubMed
-
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A. et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015;17:1601–87. - PubMed
-
- Boston Scientific Corp. Product Details, 2017. http://www.bostonscientific.com/en-US/products/defibrillators/emblem-s-i... (26 July 2019, date last accessed).
-
- Borleffs CJW, Thijssen J, de Bie MK, van Rees JB, van Welsenes GH, van Erven L. et al. Recurrent implantable cardioverter-defibrillator replacement is associated with an increasing risk of pocket-related complications. Pacing Clin Electrophysiol 2010;33:1013–9. - PubMed
-
- Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P. et al. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD Database. Circ Arrhythm Electrophysiol 2011;4:136–42. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

