The Balance of Stromal BMP Signaling Mediated by GREM1 and ISLR Drives Colorectal Carcinogenesis
- PMID: 33197448
- PMCID: PMC7617122
- DOI: 10.1053/j.gastro.2020.11.011
The Balance of Stromal BMP Signaling Mediated by GREM1 and ISLR Drives Colorectal Carcinogenesis
Erratum in
-
Correction.Gastroenterology. 2021 Nov;161(5):1728. doi: 10.1053/j.gastro.2021.09.038. Epub 2021 Sep 22. Gastroenterology. 2021. PMID: 34562358 No abstract available.
Abstract
Background & aims: Cancer-associated fibroblasts (CAFs), key constituents of the tumor microenvironment, either promote or restrain tumor growth. Attempts to therapeutically target CAFs have been hampered by our incomplete understanding of these functionally heterogeneous cells. Key growth factors in the intestinal epithelial niche, bone morphogenetic proteins (BMPs), also play a critical role in colorectal cancer (CRC) progression. However, the crucial proteins regulating stromal BMP balance and the potential application of BMP signaling to manage CRC remain largely unexplored.
Methods: Using human CRC RNA expression data, we identified CAF-specific factors involved in BMP signaling, then verified and characterized their expression in the CRC stroma by in situ hybridization. CRC tumoroids and a mouse model of CRC hepatic metastasis were used to test approaches to modify BMP signaling and treat CRC.
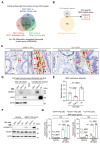
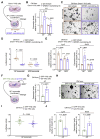
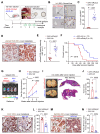
Results: We identified Grem1 and Islr as CAF-specific genes involved in BMP signaling. Functionally, GREM1 and ISLR acted to inhibit and promote BMP signaling, respectively. Grem1 and Islr marked distinct fibroblast subpopulations and were differentially regulated by transforming growth factor β and FOXL1, providing an underlying mechanism to explain fibroblast biological dichotomy. In patients with CRC, high GREM1 and ISLR expression levels were associated with poor and favorable survival, respectively. A GREM1-neutralizing antibody or fibroblast Islr overexpression reduced CRC tumoroid growth and promoted Lgr5+ intestinal stem cell differentiation. Finally, adeno-associated virus 8 (AAV8)-mediated delivery of Islr to hepatocytes increased BMP signaling and improved survival in our mouse model of hepatic metastasis.
Conclusions: Stromal BMP signaling predicts and modifies CRC progression and survival, and it can be therapeutically targeted by novel AAV-directed gene delivery to the liver.
Keywords: Bone Morphogenetic Protein; Cancer-Associated Fibroblasts; Colorectal Cancer; Tumor Microenvironment.
Copyright © 2021 AGA Institute. All rights reserved.
Conflict of interest statement
These authors disclose the following: Gareth Davies is a researcher at UCB Pharma (Slough, Berkshire, UK); A GREM1-neutralizing antibody was generated by UCB Pharma and provided to researchers at the Gut Cancer Group, the University of Adelaide (Australia), at no cost. Leszek Lisowski has consulted on technologies addressed in this paper and has stock and/or equity in companies with technology broadly related to this paper. The remaining authors disclose no conflicts.
Figures




References
-
- Kobayashi H, Enomoto A, Woods SL, et al. Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2019;16:282–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

