d-Serine Intervention In The Medial Entorhinal Area Alters TLE-Related Pathology In CA1 Hippocampus Via The Temporoammonic Pathway
- PMID: 33197499
- PMCID: PMC7796904
- DOI: 10.1016/j.neuroscience.2020.10.025
d-Serine Intervention In The Medial Entorhinal Area Alters TLE-Related Pathology In CA1 Hippocampus Via The Temporoammonic Pathway
Abstract
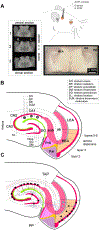
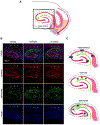
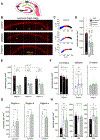
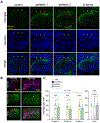
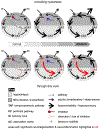
Entrainment of the hippocampus by the medial entorhinal area (MEA) in Temporal Lobe Epilepsy (TLE), the most common type of drug-resistant epilepsy in adults, is believed to be mediated primarily through the perforant pathway (PP), which connects stellate cells in layer (L) II of the MEA with granule cells of the dentate gyrus (DG) to drive the hippocampal tri-synaptic circuit. Using immunohistochemistry, high-resolution confocal microscopy and the rat pilocarpine model of TLE, we show here that the lesser known temporoammonic pathway (TAP) plays a significant role in transferring MEA pathology to the CA1 region of the hippocampus independently of the PP. The pathology observed was region-specific and restricted primarily to the CA1c subfield of the hippocampus. As shown previously, daily intracranial infusion of d-serine (100 μm), an antagonist of GluN3-containing triheteromeric N-Methyl d-aspartate receptors (t-NMDARs), into the MEA prevented loss of LIII neurons and epileptogenesis. This intervention in the MEA led to the rescue of hippocampal CA1 neurons that would have otherwise perished in the epileptic animals, and down regulation of the expression of astrocytes and microglia thereby mitigating the effects of neuroinflammation. Interestingly, these changes were not observed to a similar extent in other regions of vulnerability like the hilus, DG or CA3, suggesting that the pathology manifest in CA1 is driven predominantly through the TAP. This work highlights TAP's role in the entrainment of the hippocampus and identifies specific areas for therapeutic intervention in dealing with TLE.
Keywords: Hippocampus; Neurodegeneration; Perforant pathway; Temporal lobe epilepsy; Temporoammonic pathway; d-Serine intervention.
Copyright © 2020 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Andersen P, Bliss TV, Skrede KK (1971), Lamellar organization of hippocampal pathways. Exp Brain Res 13:222–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

