Evaluating patient-specific neoadjuvant regimens for breast cancer via a mathematical model constrained by quantitative magnetic resonance imaging data
- PMID: 33197744
- PMCID: PMC7677708
- DOI: 10.1016/j.neo.2020.10.011
Evaluating patient-specific neoadjuvant regimens for breast cancer via a mathematical model constrained by quantitative magnetic resonance imaging data
Abstract
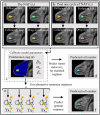
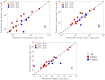
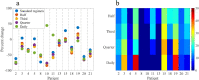
The ability to accurately predict response and then rigorously optimize a therapeutic regimen on a patient-specific basis, would transform oncology. Toward this end, we have developed an experimental-mathematical framework that integrates quantitative magnetic resonance imaging (MRI) data into a biophysical model to predict patient-specific treatment response of locally advanced breast cancer to neoadjuvant therapy. Diffusion-weighted and dynamic contrast-enhanced MRI data is collected prior to therapy, after 1 cycle of therapy, and at the completion of the first therapeutic regimen. The model is initialized and calibrated with the first 2 patient-specific MRI data sets to predict response at the third, which is then compared to patient outcomes (N = 18). The model's predictions for total cellularity, total volume, and the longest axis at the completion of the regimen are significant within expected measurement precision (P< 0.05) and strongly correlated with measured response (P < 0.01). Further, we use the model to investigate, in silico, a range of (practical) alternative treatment plans to achieve the greatest possible tumor control for each individual in a subgroup of patients (N = 13). The model identifies alternative dosing strategies predicted to achieve greater tumor control compared to the standard of care for 12 of 13 patients (P < 0.01). In summary, a predictive, mechanism-based mathematical model has demonstrated the ability to identify alternative treatment regimens that are forecasted to outperform the therapeutic regimens the patients clinically. This has important implications for clinical trial design with the opportunity to alter oncology care in the future.
Keywords: Chemotherapy; DCE-MRI; DW-MRI; Forecasting; Simulation.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Arnaout A., Lee J., Gelmon K., Poirier B., Lu F.I., Akra M., Boileau J.F., Tonkin K., Li H., Illman C. Neoadjuvant therapy for breast cancer:updates and proceedings from the seventh annual meeting of the canadian consortium for locally advanced breast cancer. Curr Oncol. 2018;25(5):e490–e498.
-
- Pinkel D. The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res. 1958;18(7):853–856. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

