Hydroxypropyl Methylcellulose E15: A Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer
- PMID: 33198094
- PMCID: PMC7696250
- DOI: 10.3390/polym12112666
Hydroxypropyl Methylcellulose E15: A Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer
Abstract
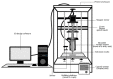
Extrusion-based 3D printing technology is a relatively new technique that has a potential for fabricating pharmaceutical products in various dosage forms. It offers many advantages over conventional manufacturing methods, including more accurate drug dosing, which is especially important for the drugs that require exact tailoring (e.g., narrow therapeutic index drugs). In this work, we have successfully fabricated phenytoin-loaded orodispersible films (ODFs) through a syringe extrusion 3D printing technique. Two different grades of hydroxypropyl methylcellulose (HPMC E5 and HPMC E15) were used as the film-forming polymers, and glycerin and propylene glycol were used as plasticizers. The 3D-printed ODFs were physicochemically characterized and evaluated for their mechanical properties and in vitro disintegration time. Then, the optimum printed ODFs showing good mechanical properties and the fastest disintegration time were selected to evaluate their drug content and dissolution profiles. The results showed that phenytoin-loaded E15 ODFs demonstrated superior properties when compared to E5 films. It demonstrated a fast disintegration time in less than 5 s and rapidly dissolved and reached up to 80% of drug release within 10 min. In addition, it also exhibited drug content uniformity within United States Pharmacopeia (USP) acceptable range and exhibited good mechanical properties and flexibility with low puncture strength, low Young's modulus and high elongation, which allows ease of handling and application. Furthermore, the HPMC E15 printing dispersions with suitable concentrations at 10% w/v exhibited a non-Newtonian (shear-thinning) pseudoplastic behavior along with good extrudability characteristics through the extrusion nozzle. Thus, HPMC E15 can be applied as a 3D printing polymer for a syringe extrusion 3D printer.
Keywords: 3D printing; hydroxypropyl methylcellulose; orodispersible film; phenytoin; syringe extrusion 3D printing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cerda J.R., Arifi T., Ayyoubi S., Knief P., Ballesteros M.P., Keeble W., Barbu E., Healy A.M., Lalatsa A., Serrano D.R. Personalised 3D Printed Medicines: Optimising Material Properties for Successful Passive Diffusion Loading of Filaments for Fused Deposition Modelling of Solid Dosage Forms. Pharmaceutics. 2020;12:345. doi: 10.3390/pharmaceutics12040345. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

