Significance of Viral Activity for Regulating Heterotrophic Prokaryote Community Dynamics along a Meridional Gradient of Stratification in the Northeast Atlantic Ocean
- PMID: 33198110
- PMCID: PMC7696675
- DOI: 10.3390/v12111293
Significance of Viral Activity for Regulating Heterotrophic Prokaryote Community Dynamics along a Meridional Gradient of Stratification in the Northeast Atlantic Ocean
Abstract
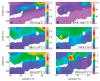
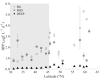
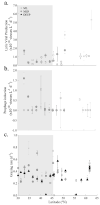
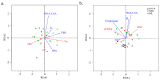
How microbial populations interact influences the availability and flux of organic carbon in the ocean. Understanding how these interactions vary over broad spatial scales is therefore a fundamental aim of microbial oceanography. In this study, we assessed variations in the abundances, production, virus and grazing induced mortality of heterotrophic prokaryotes during summer along a meridional gradient in stratification in the North Atlantic Ocean. Heterotrophic prokaryote abundance and activity varied with phytoplankton biomass, while the relative distribution of prokaryotic subpopulations (ratio of high nucleic acid fluorescent (HNA) and low nucleic acid fluorescent (LNA) cells) was significantly correlated to phytoplankton mortality mode (i.e., viral lysis to grazing rate ratio). Virus-mediate morality was the primary loss process regulating the heterotrophic prokaryotic communities (average 55% of the total mortality), which may be attributed to the strong top-down regulation of the bacterivorous protozoans. Host availability, encounter rate, and HNA:LNA were important factors regulating viral dynamics. Conversely, the abundance and activity of bacterivorous protozoans were largely regulated by temperature and turbulence. The ratio of total microbial mediated mortality to total available prokaryote carbon reveals that over the latitudinal gradient the heterotrophic prokaryote community gradually moved from a near steady state system regulated by high turnover in subtropical region to net heterotrophic production in the temperate region.
Keywords: HNA; LNA; bacterial production; carbon cycling; lysogeny; lytic infection; marine viruses; mortality; protozoan grazing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Gasol J.M., Del Giorgio P.A. Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Sci. Mar. 2000;64:197–224. doi: 10.3989/scimar.2000.64n2197. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

